Journal of the NACAA
ISSN 2158-9429
Volume 12, Issue 2 - December, 2019
Anthelmintic Resistance on Commercial Sheep Farms in the Southeastern US
- Schoenian, S. , Sheep & Goat Specialist, University of Maryland
O'Brien, D., Small Ruminant Specialist, Virginia State University
Whitley, N., Extension Specialist, Fort Valley State University
Howell, S., Laboratory Manager, University of Gerogia
ABSTRACT
In 2016, the Let’s Grow Committee of the American Sheep Industry Association funded a study to determine anthelmintic resistance on 30 commercial sheep farms in the southeastern US: Maryland (MD), Virginia (VA), and Georgia (GA). Pooled fecal samples were collected from 10 farms in each state and submitted to the University of Georgia for fecal egg count, larvae identification, and DrenchRite® larval development assay (LDA). Percentage and fecal egg count data were log transformed for analysis/inference, but LSMEANS are reported. Fecal egg count averaged 5080, 3015, and 3695 epg (SEM=487; MD=GA>VA, P<0.05), respectively for MD, VA, and GA farms. Coprocultures identified Haemonchus contortus as the predominant worm species: 89.4, 73.3, and 83.9% (SEM=3.1%; MD=GA>VA, P<0.02), respectively for MD, VA, and GA farms. All farms had high resistance to fenbendazole (SEM=9.4%). Resistance to ivermectin was similar among states, averaging 80, 100, and 100%, respectively for MD, VA, and GA. Resistance to moxidectin and levamisole was lower in MD as compared to the more southern states (P<0.04). All farms in VA and GA had resistance to three of the four anthelmintics tested. Multiple drug resistance in MD was lower, with only 80% of farms having resistance to two drugs and 60% having resistance to three drugs. The percentage of farms with resistance to all tested drugs was 0, 60, and 40%, respectively for MD, VA, and GA farms. While anthelmintic resistance varied by state and farm, all farms had resistance to at least one anthelmintic, which underscores the importance of anthelmintic resistance testing.
Introduction
Gastrointestinal parasites are the primary health problem affecting sheep raised in the warm, moist climates common to the southeastern US. The traditional method of control has been prophylactic deworming; however, the long-time use (and sometimes misuse) of dewormers has resulted in worm populations that have become increasingly resistant to treatment (O'Brien, 2018). Producers usually suspect treatment failure when clinical signs persist.
There are two ways to test for anthelmintic (dewormer) resistance. The fecal egg count reduction test (FECRT) is the “gold standard” (Coles et al., 1992). Before and after (treatment) fecal egg counts (FEC) are compared or fecal egg counts from treated and untreated (control) animals are compared. Resistance is present when the post-treatment fecal egg count reduction is less than 95% (Coles et al., 1992). Unfortunately, due to the tedious nature of doing fecal egg count reductions, few sheep producers do them.
The DrenchRite® larval development assay offers a viable alternative to fecal egg counting. From a single pooled fecal sample, it determines resistance status to all drug groups currently available to treat small ruminants in the US. However, the DrenchRite® test is expensive ($450 per sample in 2016), and most producers are reluctant to spend the money.
Materials and Methods
The Let’s Grow Committee of the American Sheep Industry Association provided funding to cost-share the DrenchRite® test for 30 commercial sheep farms in Maryland (MD), Virginia (VA), and Georgia (G A). Farms with at least 100 breeding ewes were favored for the study. Selected farms represented a variety of breeds, management systems, and climatic conditions. Co-PI’s assisted with collection of fecal samples and interpretation of results for individual farms.
A). Farms with at least 100 breeding ewes were favored for the study. Selected farms represented a variety of breeds, management systems, and climatic conditions. Co-PI’s assisted with collection of fecal samples and interpretation of results for individual farms.
In the summer of 2016, pooled fecal samples were collected from sheep farms in MD (n=10), VA (n=10), and GA (n=10) and prepared according to the DrenchRite® protocol (Howell and Storey, 2016). Samples were shipped overnight to the University of Georgia College of Veterinary Medicine Department of Infectious Diseases for analysis. Fecal egg count was determined using the Modified McMaster procedure (Coles, et al., 1992) . Eggs were isolated and placed in specialized assay plates, with doubling concentrations of drugs (Howell and Storey, 2016). After an incubation period, plates were examined to determine species and susceptibility to drugs (Howell and Storey, 2016).
Resistance status was determined for individual farms, as well as states and farms. Percentage and fecal egg count data were log transformed for analysis/inference, but LSMEANS are reported.
Results
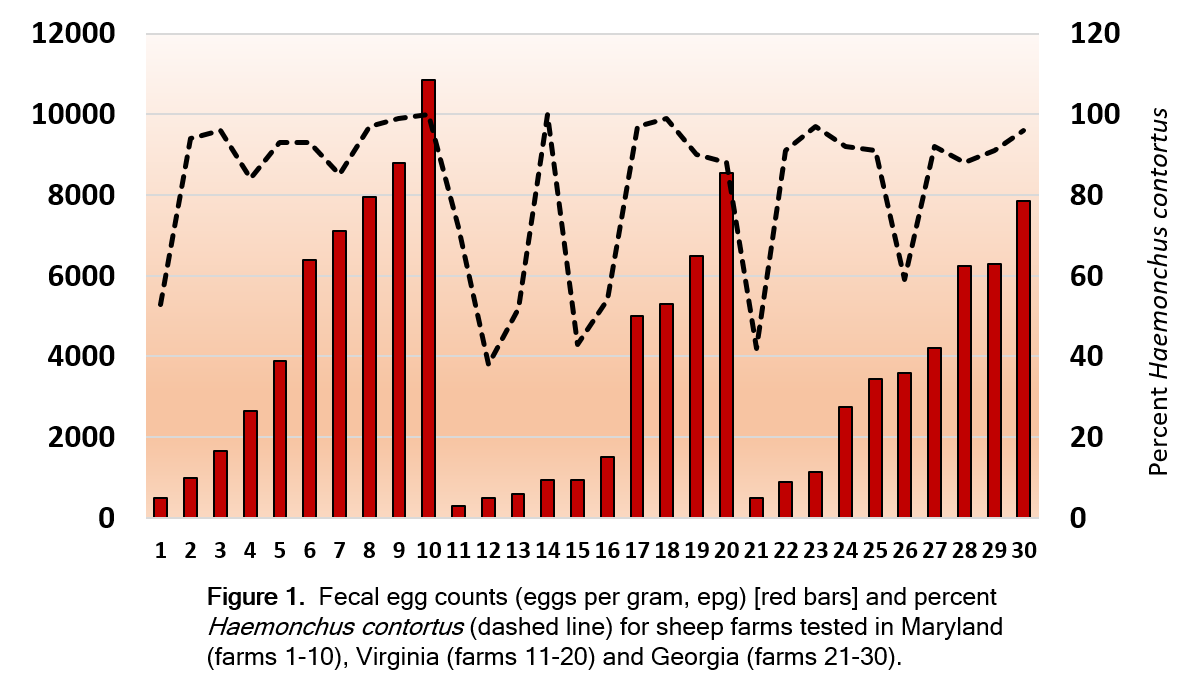
VA farms had the lowest fecal egg count (P<0.05). MD and GA farms were similar. Average fecal egg count was 5080, 3015, and 3695 epg (SEM=487), respectively for MD, VA, and GA farms (Figure 1). Haemonchus contortus was the most prevalent gastrointestinal parasite on all farms. VA farms had the lowest percentage (P<0.02). MD and GA farms were similar. Percentages averaged 89.4, 73.3, and 83.9% (SEM=3.1%), respectively for MD, VA, and GA farms (Figure 1).
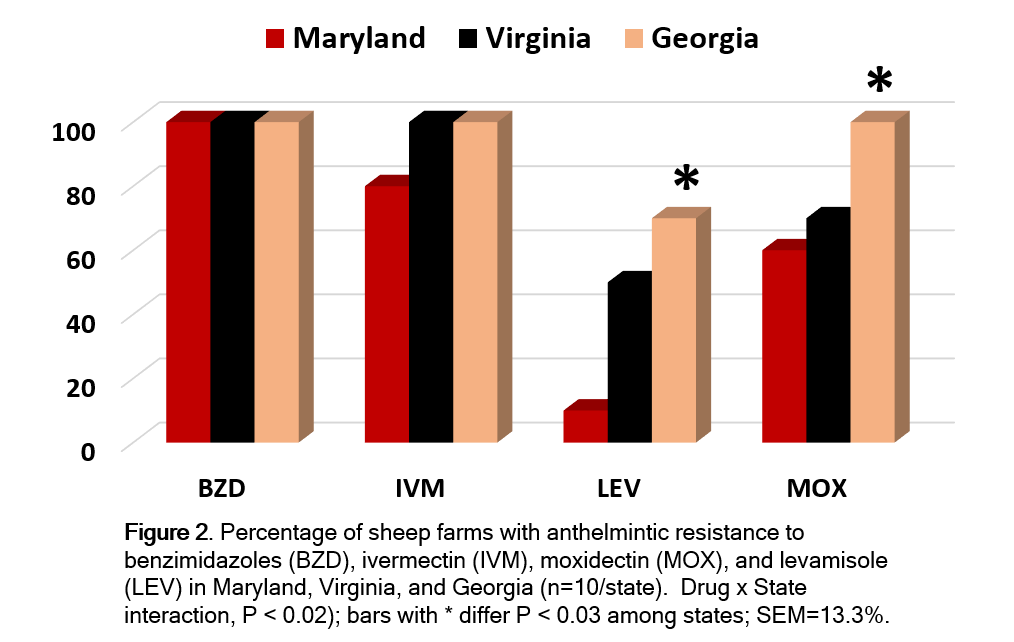
All farms had a high level of resistance to fenbendazole (SEM=9.4%) and all but two had resistance to ivermectin (Figure 2). Resistance to ivermectin was similar among states, averaging 80, 100, and 100%, respectively for MD, VA, and GA farms (Figure 2). Resistance to moxidectin and levamisole was lower (P<0.04) in MD than VA and GA (Figure 2).
All of the farms in VA and GA had resistance to three of the four anthelmintic groups tested. Multiple drug resistance in MD was lower with only 80% (SEM=3.8%) of the farms tested having resistance to two drugs and only 60% (SEM=4.7%) having resistance to three drugs.
The percentage of farms with resistance to all tested drugs was 0, 60, and 40%, respectively for MD, VA, and GA farms. While anthelmintic resistance varied by farm and state, all farms had resistance to at least one drug.
Conclusions
While anthelmintic resistance was higher in the more southern states (VA and GA), all farms had resistance to at least one dewormer.
The results indicate the need for sheep producers to test for anthelmintic resistance and to follow best management practices to minimize the need for deworming and prolong the effectiveness of existing drug resources.
For many farms, combination treatments will be necessary to effectively treat clinical parasitism, as treatment with individual dewormers may be insufficient to alleviate clinical symptoms (Kaplan, 2017).
Literature Cited
Coles, G.C., Bauer, C., Borgsteede, F.H.M., Geerts, S., Klei, T.R., Taylor, M.A., and Waller, P.J. (1992). World Association for Advancement of Veterinary Parasitology (W.A.A.V.P.) methods for detection of anthelmintic resistance in nematodes of veterinary importance. Vet. Parasitol. 44:35-44.
Howell, S. and B. Storey. (2016). DrenchRite Larval Development Assay for laboratory detection of anthelmintic resistance. University of Georgia College of Veterinary Medicine Department of Infectious Diseases.
Kaplan, R. (2017). Combination Treatments: The Time is Now. American Consortium for Small Ruminant Parasite Control. Retrieved 09.13.19 from https://www.wormx.info/combinations.
O’Brien, D. (2018). Managing Dewormer Resistance. Retrieved 09.13.19 from https://docs.wixstatic.com/ugd/6ef604_3981789ca4d34d74913834b1ea1b0b16.pdf.
