Journal of the NACAA
ISSN 2158-9429
Volume 13, Issue 1 - June, 2020
Abscisic Acid And Gibberellic Acid Applications Increase Cluster Looseness Without Any Major Effects On Fruit Composition Or Return Bloom Of ‘Chardonnay’ Grape In Southern New Jersey
- Gohil, H. L., Agriculture And Natural Resource Agent, Rutgers University Cooperative Extension
Ward D., Extension Specialist, Rutgers University New Jersey Agriculture Experiment Station
ABSTRACT
Abscisic Acid (ABA) and Gibberellic Acid (GA3) effects on cluster looseness, fruit set and fruit composition in Vitis vinifera ‘Chardonnay’ were studied at the Rutgers Agricultural Research and Extension Center in southern New Jersey in 2015 and 2016. A randomized complete block experimental design was employed with five blocks of six treatments. The same vines were subjected to the same treatments in both years. Treatments were comprised of untreated control, foliar sprays of (S)-cis-ABA (S-ABA) at 100 ppm or 200 ppm applied at two different times during bloom, and GA3 applied once at single rate. Cluster looseness was significantly increased by both S-ABA and GA3 treatments in both years. The control had the tightest clusters compared to all other treatments, however, rate and number of S-ABA applications did not differentially affect cluster looseness. Components of cluster architecture and fruit composition were not consistent during the two years. There was no clear trend that cluster weight, berries per rachis length, number of shot or normal berries, total rachis or main rachis length correlated with the cluster looseness, however, the combination of factors could have resulted in cluster looseness.
Introduction
‘Chardonnay’ is one of the most consumer preferred (IRI, Wines Vines Analytics, 2018), and widely planted wine grape cultivars in the US, including New Jersey. It is adapted to cool climates such as found in the northeastern United States. Though 2015 and 2016 were relatively drier growing seasons, wet and humid summers are common in New Jersey (Ludlum, 1982). In high humidity and still air conditions fungi such as Botrytis cinerea thrive and cause rapid deterioration of the fruit. Infections of Botrytis cinerea can occur as early as bloom, and its disease expression (Keller et al., 2003), a bunch rot, is difficult to manage in tight-clustered cultivars where spray coverage inside the cluster gets restricted (Hed et al. 2009; Vail et al., 1998). Under most conditions Botrytis infected bunches or Botrytis Bunch Rots (BBR) result in poor quality wine through modified chemical composition of infected berries resulting in simple sugars being converted into glycerol and gluconic acids and polysaccharides which hinder the clarification of wine (Wilcox, 2015).
Fungicide application has been one of the most effective methods of managing BBR, it however requires season-long spray programs which can substantially increase production costs (Hed et al, 2009). Several studies have reported the effectiveness of longer rachises and more open bunches on reducing BBR symptoms (Hed et al., 2009; Mundy et al., 2014; and Roberto et al., 2015). Early leaf removal (Hed et al., 2015) results in cluster elongation and subsequent reduction in BBR incidence. Such cultural practices require leaf removal around the clusters, before fruit set. Leaf removal reduced incidence of BBR in ‘Vignoles’ and ‘Pinot Gris’ clusters, however the reduction was less than 50% of what was achieved by fungicide applications (Ferree et al., 2003). Also, even if the cost is low enough to make early leaf removal economically feasible, some growers may consider it risky, and will be hesitant to remove photosynthesizing leaves that contribute to berry development. Additionally, late season leaf removal can cause poor berry skin quality by sun burn from over exposure (Vance et al., 2013).
Gibberellic acid (GA3) is perhaps the only plant growth regulator (PGR) tested extensively for reducing bunch compactness with the aim of reducing BBR in wine and table grapes. The established practice of making GA3 applications at bloom or post bloom to reduce bunch compactness has met with varying success, and sometimes conflicting results (Christodoulou et al., 1968; Dass and Randhawa, 1968; Hed et al., 2011; Spring and Viret, 2009; Weaver and Pool, 1971). Several factors, that are not well understood, predict the outcome of GA3 application including differential sensitivity of different cultivars to GA3 and response under humid climate growing conditions. Major concerns for using GA3 in wine grape cultivars has been poor return bloom and non-uniform sized berries (Hed et al., 2009). These are likely reasons for low adoption of GA3 in wine grapes to reduce bunch compactness.
Abscisic acid (ABA), a naturally occurring plant hormone, is involved in multiple pathways of plant metabolism and generally plays an inhibitory role such as inhibiting cell division, preventing root development in saline soils, inducing seed dormancy, inhibiting seed germination, and inhibiting fruit ripening (Salisbury and Ross, 1978). There have been several reports on effects of exogenous application of an isomer of this plant growth regulator, (S)-cis-abscisic acid (S-ABA) mainly in table grape (Peppi et al., 2006). However, there are a few reports of the effects of exogenous application of S-ABA on wine grapes where it could either improve spring bud cold-hardiness in Merlot (Bowen et al., 2016) or autumn bud freeze tolerance in Chardonnay (Dami et al., 2015). Weaver and Pool (1969) found that 1000 ppm of ABA led to significant decrease in the firmness. Recently, Padmalatha et al. (2017) reported the effects of exogenous S-ABA application at 150 and 300 ppm concentration, during bloom and fruit set, on berry numbers, rachis length, and cluster uniformity in seedless table grape variety ‘Early Sweet’. However, there are no known reports of ABA on fruit set or cluster looseness in wine grape varieties. In the present study, we examined the effect of application of S-ABA on clusters of ‘Chardonnay’ grapes to see if 100 and 200 ppm rates of S-ABA applied either once or twice near the time of full bloom increased cluster looseness. Due to known effects in ‘Chardonnay’, GA3 was used as a positive control.
Materials and Methods
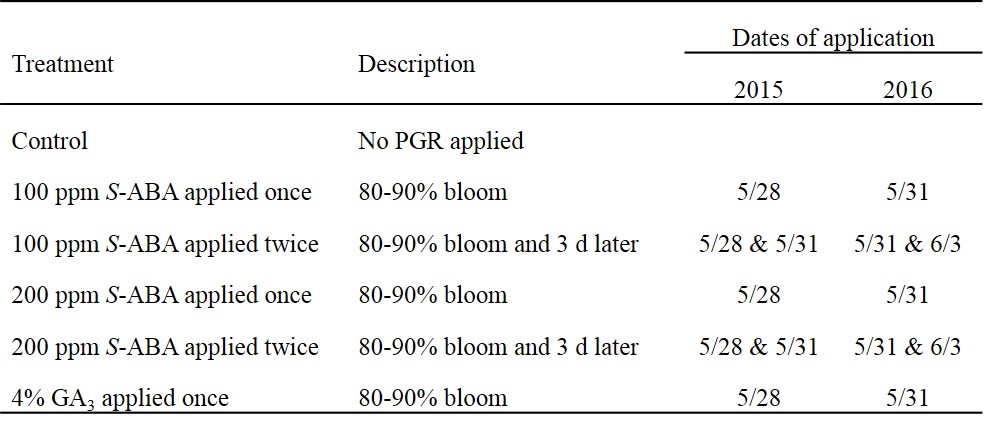
Vineyard description. In both years, 2015 and 2016, the trial was conducted in a ‘Chardonnay’ vineyard at Rutgers Agricultural Research and Extension Center in Upper Deerfield (39.4949°N; 75.2169°W), New Jersey. Soil series is Sassafras sandy loam (mesic typic Hapludults) with 2-5% slopes, in the Outer Coastal Plain American Viticultural Area. Vines were 11 and 12 years old on 101-14 (Millardet et de Grasset) rootstock with spacing of 5.5 ft. between vines and 9 ft between rows. The vines were trained to a standard bi-lateral cordon system using vertical shoot positioning and drip irrigation. A randomized complete block experimental design was employed with five blocks of six treatments (Table 1). The experimental vineyard was managed uniformly using standard cultural practices (Wolf, 2008).
PGR Treatment application. Each treatment plot consisted of a single vine flanked by guard vines on each side. Vines with uniform canopy development were selected for inclusion in the study before allocation of treatments. The same vines were subjected to the same treatments during both the years. The 80% bloom timing was defined as when approximately 80% of the flowers had dropped their caps (calyptras) and similarly 100% bloom was defined when approximately ~100% of caps had dropped from the flowers (Table 1). The S-ABA formulation was ProTone® SG (Valent BioSciences Corporation, Libertyville, IL, USA), a commercially available granular formulation, and the GA3 formulation was ProGibb 4% (Valent BioSciences Corporation, Libertyville, IL, USA). The S-ABA treatments were the novel treatments of interest, GA3 was used as a positive control, and a non-treated control was included. For each treatment, the amount of product and volume of spray solution was determined based on rates of application during preliminary sprays on non-experimental vines. Products were readily dissolved in water and applied without added surfactant using the label direction. Whole vines were sprayed to ensure full coverage of canopy and flowers using a CO2 pressurized backpack sprayer. Non-treated control vines were not sprayed. The label directions specified for application of ProTone® SG and ProGibb 4% were followed. These products are already registered for use in table grapes in the state of New Jersey.
Table 1. Description of plant growth regulator treatments, Abscisic Acid (S-ABA) and Gibberellic Acid (GA3), and their application timing on ‘Chardonnay’ grapevines in southern New Jersey.
Phyto-toxicity measurements. Two weeks after PGR application leaf phytotoxicity was assessed using a 0 to 5 scale where; 0 = no phytotoxicity; 1 = less than 10% or slight leaf chlorosis or discoloration; 2 = 11-30% leaf chlorosis or discoloration; 3 = 31-60% leaf chlorosis or discoloration; 4 = 61-90% leaf chlorosis or necrosis with minor defoliation and; 5 ≥ 90% leaf chlorosis or necrosis with major defoliation.
Return bloom. One major concern for the safety of applying PGRs, especially GA3, is the potential for negative effects on return bloom. In the year after application of the treatments, return bloom was evaluated in spring 2016 and 2017 by counting the number of flowers clusters per plot.
Cluster looseness measurements. For physicochemical analysis of grapes, all the clusters from each treated plot were harvested when the grapes reached technological maturity. Samples were stored in a cold chamber and maintained at 0°C until further analysis. Though all the clusters per plot were harvested, fruit was not weighed for the yield as the main objective was to determine the cluster looseness. Then sixteen randomly selected clusters from each harvested plot were sampled for the harvest measurements. Clusters were rated for looseness using a rating scale adapted from Zabadal and Dittmer (1998; Figure 1). Following the looseness measurements, all sixteen clusters were weighed for the average cluster weight measurement. All the berries were detached from each cluster and then shot berries and normal berries were counted. Normal berries are all the berries which were ripe or soft and have changed color. Shot berries were defined as very small, hard and green berries where growth stopped at the ovary stage (Fougere-Ritof et al., 1995; Keller, 2012). For each cluster, total berries equaled the sum of normal and shot berries. Percent normal berries was the ratio of normal berries to total berries while percent shot berries was the ratio of shot berries to total berries. Cluster architecture measurements such as main rachis length (cm) and total rachis length (sum of main and each lateral rachis length) were measured. For each plot 100 random berries were used to get average berry weight. Finally, total berry weight per rachis length (g cm-1) was determined.

Fig. 1. Typical ‘Chardonnay’ clusters with each cluster looseness rating; 1 = almost all berries touching each other; 2 = more than half of berries touching each other; 3 = a few berries touching each other; 4 = most not touching with a few gaps into the center of the cluster; 5 = most berries are separate from each other with much of rachis clearly visible.
Fruit primary chemistry measurements. To measure soluble solids, pH, and titratable acidity (TA), four clusters from harvested clusters of each treatment replicate, were sampled randomly. For analysis, these four clusters were crushed into a single juice sample, using a muslin cloth. The total soluble solids (°Brix) were measured with a digital handheld refractometer (PR-32 Pallette; Atago U.S.A., Bellevue, WA). TA and pH were measured on an automated titrator (Titralab 840, Radiometer Analytical, Lyon, France); titration was to a pH endpoint of 8.2, using 0.1 N sodium hydroxide (Illand et al., 2000).
Fruit set. To understand if cluster looseness was caused by reduced fruit set, it was measured in 2016. Fruit set was calculated as percentage of flowers in a cluster that formed berries. Two randomly selected flower clusters per plot were selected before bloom. Open flowers were counted carefully while on the vine. At harvest, the same clusters were collected and individual berries per cluster were counted.
Statistical analyses. For all measured responses, the initial analysis was performed using an ANOVA model that included the effects of year, treatment, and the year by treatment interaction as fixed effects. The interaction of year with treatment did not explain substantial variation, so it was removed and a reduced model containing year and treatment, was used for analyzing all responses for both years together. Treatment means were compared using Fisher’s protected LSD. Non-significant (P>0.05) treatment effects on response variables (i.e. Total Soluble Solids (TSS), Total Titratable Acidity (TTA), pH, and return bloom flower cluster number) which we were concerned may be adversely affected by treatment were further characterized by calculating the 95% confidence interval on differences between treatment means. We have presented the half-interval for these responses to indicate the minimum detectable difference, which is the size of effect that our study would have been able to detect as a significant difference. All statistical calculations were performed using the GLIMMIX procedure of the SAS system (ver 9.4, SAS institute, Cary, NC).
Results and Discussion
Treatment effects on cluster looseness. Both S-ABA and GA3, significantly reduced cluster compactness compared to the control (P < 0.001). The GA3 caused the loosest clusters and the control had the tightest clusters, though GA3 treated clusters had non-uniform berries compared to all other treatments (Table 2). There was an incremental trend of S-ABA where the higher concentration (200 ppm) resulted in relatively looser clusters compared to 100 ppm treatment, however there was no significant difference. GA3 treated clusters were numerically rated looser than all the S-ABA treatments, however, there was no significant difference among PGR treatments. Our results concur with the recent report (Padmalatha et al., 2017) where clusters treated with S-ABA at pre-bloom and full bloom were significantly loosened compared to control during all three years of experiments. The results concur with previous research demonstrating that GA3 reduces the cluster compactness in ‘Chardonnay’ (Christodoulou et al. 1968; Hed, 2011). The lack of uniformly sized berries (data not collected) is an observation (personal communication with Bryan Hed) that has not been reported in previous research. Rate and timing of S-ABA application did not have any significant effect on cluster looseness.
Table 2. Means averaged over two years of cluster looseness ratingz, percentage shot berries, cluster weight, main rachis length, total rachis length, and berry weight per cm rachis length in response to different rates and timings of abscisic acid (S-ABA) and gibberellic acid (GA3) applied at bloom to Chardonnay grapevines in New Jersey in 2015 and 2016.
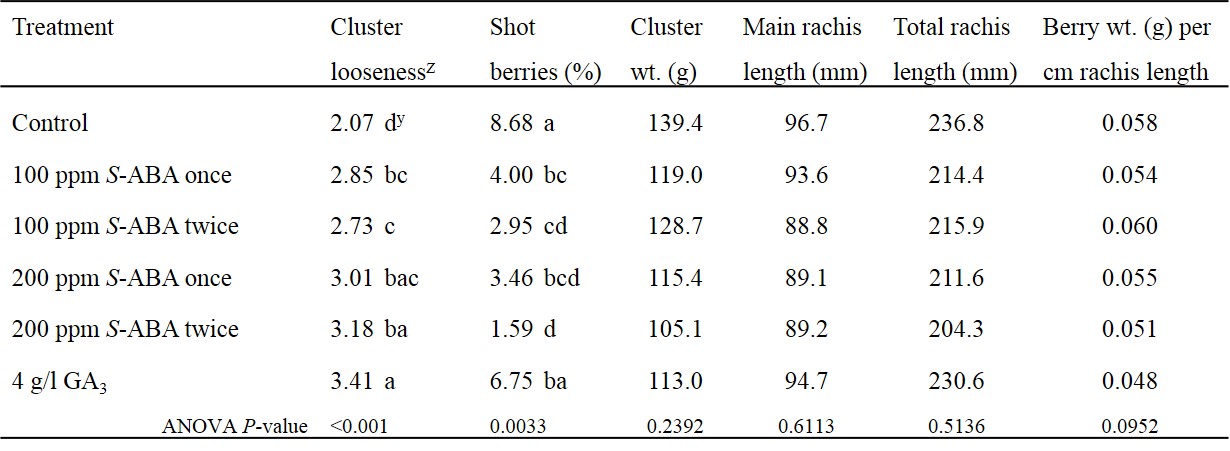
Z visual ratings where 1 = almost all the berries are touching each other; 2 = more than half of the berries touching each other; 3 = very few berries touching each other and with minimal surface area; 4 = almost half of the berries are separate from each other with few gaps; 5 = most of the berries are separate from each other with visible rachis
Y means in a column with no letters in common are significantly different at α = 0.05 according to Fisher’s Protected LSD.
S-ABA significantly reduced percent shot berries (P = 0.0033) compared to control. The difference between control and the double application of S-ABA at higher concentration was substantial. Percentage shot berries in control and GA3 treated vines were comparable (Table 2). In a similar study, Padmalatha et al. (2017) found that S-ABA application at 50% bloom and full bloom stage significantly increased the bigger berries fraction and, decreased the smaller berries fraction (<12 mm). Reduced percentage of shot berries in 2015, but not in 2016 (data not shown) could be due to temperature effects. In general, the spring of 2015 was cooler than 2016. For example, though the maximum temperatures during four days of PGR treatments averaged 83° F in 2015 and 89° F in 2016, average temperature five days after full bloom was 70° F in 2015 and 82° F in 2016 (Rutgers NJ Weather Network). The role of S-ABA in causing shot berries has not been explored, however, the inhibitory effects of S-ABA in various physiological processes are well documented. Role of exogenous S-ABA in abscission of flowers and young developing fruits such as apple (Edgerton, 1971; Greene et al., 2011), and cherry (Zucconi et al., 1969) is known. The role of ABA as a down regulator after pollination and during grape berry formation, either to protect the ovary tissue or to prevent fruit development before pollination and fertilization has been proposed (Vriezen et al., 2008).
Average cluster weight of PGR treatments was numerically lower compared to control, but the difference was not significant (P = 0.2392). Similarly, main rachis length and total rachis length was comparable among all the treatments (Table 2). Padmalatha et al. (2017) found that following the S-ABA treatment, rachis length was comparable to control at full bloom in two out of three years. Though GA3 application is known to reduce cluster weight and increase cluster length by rachis elongation, the differences compared to control have not always been significant (Ferree et al, 2003). PGRs did not change the average berry weight per cm of rachis length (Table 2; P= 0.0952). One may wonder why PGRs did not affect the number of berries per cm of rachis length while cluster looseness was quite evident during both the years. Reduced berry weight per rachis length would have been a straightforward explanation for the cluster looseness, however, rachis length itself does not respond consistently to ABA as reported by Padmalatha et al. (2017). Factors such as the size of berries and reduction in shot berries could have also influenced the cluster looseness.
Treatment effects on return bloom, fruit set, phytotoxicity and fruit chemistry. Average number of flowers per vine (return bloom) were comparable in all treatments (P=0.5301; Table 3). Fruit set was comparable for all treatments in 2016 (P=0.7926) and no such data were recorded in 2015 (data not shown). Visual ratings of vines detected no phytotoxicity due to S-ABA or GA3 application (data not shown). Also, total soluble solids, pH and TA were also not affected by PGRs (Table 3).
Table 3. Means averaged over two years of return bloom, juice pH, juice total titratable acidity (TTA), and juice total soluble solids (TSS) in response to abscisic acid (S-ABA) and gibberellic acid (GA3) applied at bloom to ‘Chardonnay’ grapevines in New Jersey in 2015 and 2016.
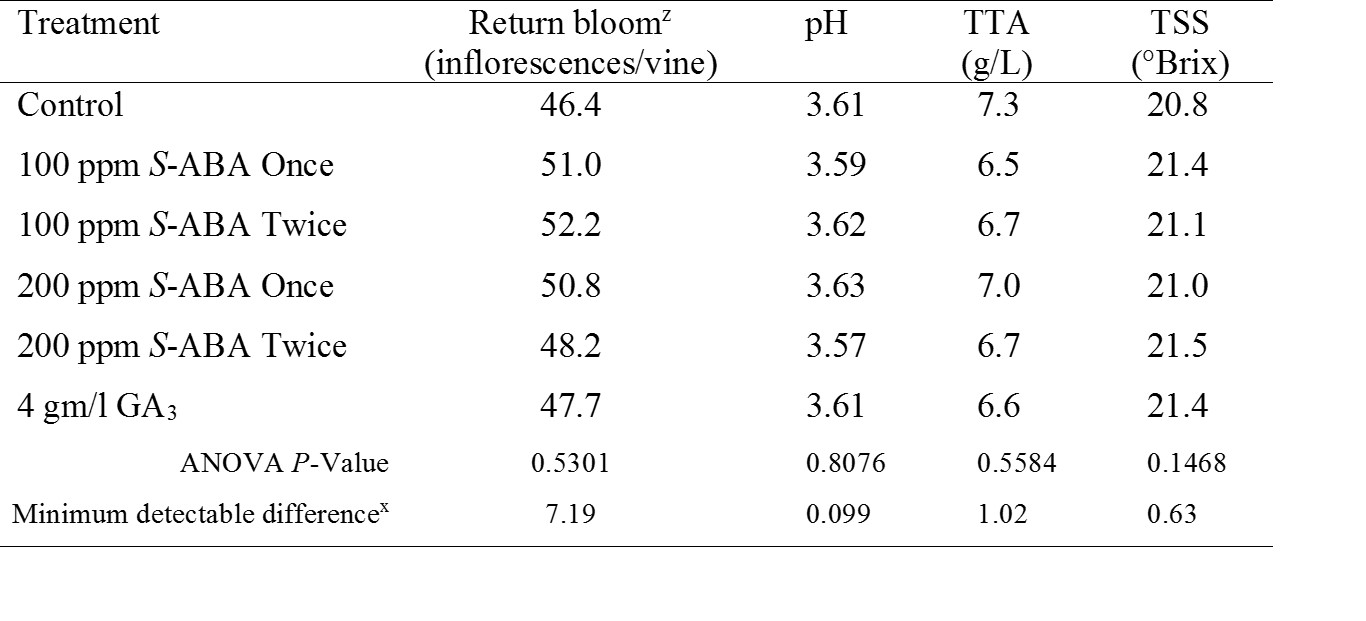
z Return bloom counts are following 2015 and 2016 season and were determined in 2016 and 2017.
y Means within a column with no letters in common are significantly different at α = 0.05 according to Fisher’s Protected LSD.
x The minimum detectable difference is one half of the 95% confidence interval on the difference between any two means.
Conclusions
In conclusion, the cluster looseness was significantly increased by PGR treatments. Interestingly, the berries per cm of rachis length were similar among all treatments during both the years. The pattern of average berry weight, percentage normal, percentage shot berries, rachis length, and total length were not consistent during both years when considered as individual response variables. However, the combination of these factors may have resulted in increased cluster looseness.
Acknowledgements
The authors thank Jeff Hammerstedt and Dawn Baruffi for technical help and Valent BioSciences LLC for financial support of this project.
Literature cited
Christodoulou, A. J., J.R. Weaver, and R. M. Pool. (1968). Relation of gibberellin treatment to fruit set, berry development, and cluster compactness in Vitis vinifera grapes. Journal of American Society of Horticultural Sciences 92:301-310.
Bowen P., K.C. Shellie, L. Mills, J. Willwerth, C. Bogdanoff, and M. Keller. (2016). Abscisic acid form, concentration, and application timing influence phenology and bud cold hardiness in Merlot grapevines. Canadian Journal of Plant Science 96:347-359.
Dami, I., S. Li, S., P.A. Bowen, C.P. Bogdanoff, K.C. Shellie, and J. Willwerth. (2015). Foliar applied abscisic acid increases ‘Chardonnay’ (Vitis vinifera) grapevine bud freezing tolerance during autumn cold acclimation. HortTechnology 25: 293–305
Dass, H. C., and G.S. Randhawa. (1968). Response of certain seeded Vitis vinifera varieties to gibberellin application at post-bloom stage. American Journal of Enology and Viticulture 19:56-62.
Edgerton, L.J. (1971). Apple abscission. HortScience. 6(3):378-382.
Greene, D. W., Schupp J.R. and Winzeler, H.E. (2011). Effects of abscisic acid and benzyl adenine on fruit set and fruit quality of apples. HortScience 46(4):604-609.
Hed, B., H. K. Ngugi, and J. W. Travis. (2009). Relationship between cluster compactness and bunch rot in Vignoles grapes. Plant Disease 93:1195-1201.
Hed, B., H. K. Ngugi, and J. W. Travis. (2011). Use of gibberellic acid for management of bunch rot on Chardonnay and Vignoles grapes. Plant Disease 95:269-278.
Hed, B., H. K. Ngugi, and J. W. Travis. (2015). Short- and long-term effects of leaf removal and gibberellin on Chardonnay grapes in the Lake Erie region of Pennsylvania. American Journal of Enology and Viticulture 66: 22-29.
Iland, P., A. Ewart, J. Sitters, A. Markides, and N. Bruer. (2000). Techniques for chemical analysis and quality monitoring during winemaking. Wine Promotions, Campbell Town, SA.
IRI, Wines Vines Analytics. Domestic table and sparkling wines sales in multiple-outlet and convenience stores, 52 weeks ended July 15, 2018.
Keller, M., O. Viret, and F. M. Cole. (2003). Botrytis cineria infection in grape flowers; Defense reaction, Latency, and disease expression. Phytopathology. 93:316-322.
Ludlum, David M., (1983). The New Jersey Weather Book. Rutgers University Press, New Brunswick, 25
Mundy, D.C., S. R. Haycock, V. Raw, R.H. Agnew, E. Sherman, A.G. McLachlan and Hagerty, G.C. 2014. Effects of chemical and natural product treatments on bunch openness and botrytis bunch rot in Sauvignon Blanc grapes. New Zealand Plant Prot. 67:157-167.
Padmalatha, K., H. Weksler, A. Mugzach, A.K. Acheampong, Zheng, C., Halaly-Basha, T., and Or, E. (2017). ABA application during flowering and fruit set reduces berry number and improves cluster uniformity. American Journal of Enology and Viticulture 68: 275-282.
Peppi, M.C., M.W. Fidelibus, and N. Dokoozlian. (2006). Abscisic acid application timing and concentration affect firmness, pigmentation and color of ‘Flame Seedless’ grapes. HortScience 41: 1440–1445.
Roberto S R., F.S.B. Wellington, R.C. Colombo, R. Koyama, I. Hussain and R. Teodoro de Souza. (2015). Berry-cluster thinning to prevent bunch compactness of ‘BRS Vitoria’ a new black seedless grape. Scientia Horticulturae 197: 297-303.
Salisbury F. B. and C. W. Ross. (1978). Plant Physiology: 2nd ed. Wadsworth. Belmont, CA.
Spring, J. L., and O. Viret. (2009). Influence of thinning methods on yield, bunch morphology, grey and sour rot, and wine quality of Pinot noir. Revue Suisse de Viticulture, Arboriculture, Horticulture 41:95-101.
Vail, M. E., J. A. Wolpert, W.D. Gubler and M.R. Rademacher. (1998). Effect of cluster tightness on Botrytis bunch rot in six Chardonnay clones. Plant Diseases 82:107-109.
Vriezen W.H., R. Feron, F. Maretto, J. Keijman and C. Mariani. (2008). Changes in tomato ovary transcriptome demonstrate complex hormonal regulation of fruit set. New Phytology 177, 60–76.
Weaver, R. J., A.N. Kasimatis, and S.B. McCune. (1962). Studies with gibberellin on wine grapes to decrease bunch rot. American Journal of Enology and Viticulture 13:78- 82.
Weaver, R. J., and R.M. Pool. (1971). Thinning ‘Tokay’ and ‘Zinfandel’ grapes by bloom sprays of gibberellin. American Society of Horticultural Sciences 96:820- 822.
Wilcox W. F., D. Walter, Gubler, K. Jerry and K.J. Uyemot. (2015). Compendium of Grape Diseases, Disorders, and Pests: 2nd ed. The APS, St. Paul, MN.
Wolf, T.K. (ed.). (2008). Wine Grape Production Guide for Eastern North America. Cooperative Extension NRAES-145.
Zabadal, T.J., and T.W. Dittmer. (1998). Vine management system affect yield, fruit quality, cluster compactness, and fruit rot of ‘Chardonnay’ grape. HortScience 33(5):806-809.
Zucconi, F., R. Strosser, and M. J. Bukovac. (1969). Promotion of fruit abscission with abscisic acid. Bioscience 19:815-817.
