Journal of the NACAA
ISSN 2158-9429
Volume 13, Issue 2 - December, 2020
Soil Myth Busting for Extension Educators: Reviewing the Literature on Soil Nutrition
- Chalker-Scott, L. , Extension Specialist And Associate Professor, Washington State University
Downer, A.J., Farm Advisor, University of California
ABSTRACT
Home gardening is increasingly popular, particularly during a global pandemic when many people are confined to home and are spending more time outside. Novice and experienced gardeners alike are likely to access agricultural production information that is not necessarily relevant to their home gardens or landscapes. Specifically, there are soil fertility guidelines intended for intensive, monocultural crop production that can harm plants, soil biota, and nearby aquatic systems when applied to a home garden situation. This article will address six common misperceptions about managing soil nutrition in nonagricultural situations.
INTRODUCTION
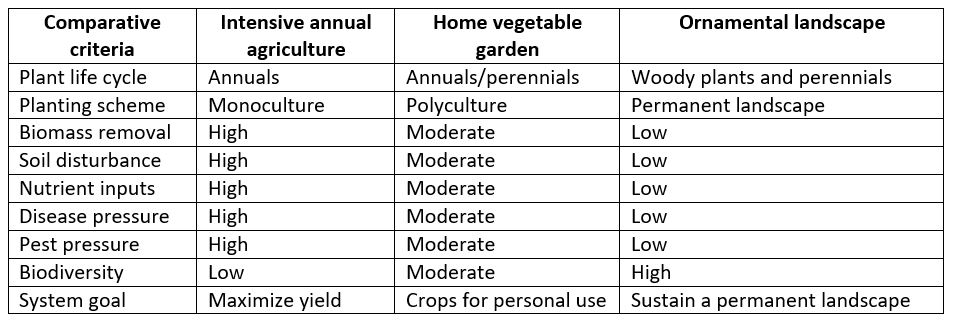
There is a robust literature, spanning many decades, on managing soil for agricultural crop production. Conversely, the field of urban horticulture is relatively young and literature regarding soil nutrient management in nonagricultural situations is comparatively sparse, though rapidly expanding. What is often eye-opening for gardeners and extension educators alike is the realization that soil nutrient management for a monocultural annual crop is dramatically different from what is needed for home gardens and ornamental landscapes (Figures 1a-c). One of the chief differences among these systems is that agricultural crop growth is optimized for maximum yield, which is not necessarily the primary goal in a home garden and certainly not a goal at all in an ornamental landscape. Instead, a primary goal in both of the latter systems is to maximize plant and soil health in an aesthetically pleasing manner.
Figures 1a, b and c. Monocultural crop growth (a) requires different nutrient management than either home vegetable gardens (b) or landscapes (c). Lawns, such as the one seen on the right, can be a mix of groundcovers and not a monocultural turf. Photo 1a by Snappy Goat; 1b and 1c by Linda Chalker-Scott.
The criteria for managing these systems are compared in Table 1.
Table 1. Characteristics of conventional agricultural plots, home vegetable gardens, and ornamental landscapes.
Agricultural crop production entails removal of most, if not all, of the plant material on site. Soil nutrient levels drop as crop yields are taken, and inputs of fertilizers and/or organic matter are required. In annual cropping systems, annual tillage is used to incorporate crop residues or other amendments – but this destroys soil structure and is not recommended (Chalker-Scott and Downer, 2019). This scenario is not typical, however, for a home vegetable garden and certainly not for an ornamental landscape. Yet many soil nutrition recommendations made for home garden and landscape situations are based on agricultural crop production and concurrent nutrient deficiencies. Misapplying this information to nonagricultural situations can lead to gardeners overapplying fertilizers and organic material to levels that are unnaturally high and create nutrient imbalances and toxicities that can damage soil, plant, and adjacent aquatic systems health (Erickson et al., 2005; Warner et al., 2018).
Previously, we have deconstructed several soil myths related to soil structure and function (Chalker-Scott and Downer, 2019). This literature review expands the discussion to soil myths related to nutrient status. The purposes this review are:
- to identify some popular misperceptions about managing nutrients in nonagricultural soils;
- to provide a brief, science-based explanation on why these beliefs are not accurate;
- to provide links to published, peer-reviewed citations that support the explanations and can be distributed to clientele; and
- to describe sustainable methods of managing soil fertility in nonagricultural soils based on current and relevant applied soil sciences.
Myth 1: “Incorporate plenty of organic matter to create a healthy soil”
In our previous article (Chalker-Scott and Downer, 2019) we discussed the damage done to soil structure and function through incorporation of amendments. Not only does incorporation of amendments into the planting site reduce successful plant establishment, it also rapidly changes soil nutrient load. The addition of organic matter to the soil not only increases the percentage of organic matter in the soil, it increases the concentration of a number of plant nutrients and other inorganic chemicals. Both of these changes can have negative impacts on soil, plant, and adjacent ecosystem health.
- Increased percent organic matter content. Every soil system, if allowed to come to equilibrium, contains a sustainable amount of organic material. (This does not include any material on top of the soil, i.e., a mulch, but only that found in the profile). This equilibrium represents the net amount of organic material that remains after additions (from soil biota death and from natural incorporation by earthworms, for example) and subtractions (from use by plants, detritivores, and microbes). Percent organic matter varies widely among different environments, but in general the number is quite low – in the single digits. Soil scientists are often loath to set a normal range for soil organic material, instead preferring to explain it is as a very small piece of the soil pie. In fact, a pie chart is the easiest way to visualize what soil contains.
Ideal soils are generally half solids and half pore space, with the pore space further divided into macropores (filled with air) and micropores (filled with water) (Figure 2). The solid portion includes rock, sand, silt, and clay, and well as organic matter. The organic matter supplies most of the nutrients to the soil, but it is generally a minor fraction of the “solids” portion. A stable landscape soil will have a percent organic matter that remains relatively consistent year to year.
Figure 2. A pie chart with approximate volumes of solid materials, water, and air.
How can you determine the sustainable percentage of organic matter in a soil? A series of annual soil tests from a university or commercial lab, including an organic matter determination as well as the nutrient analysis, can answer this question. (It would be important to refrain from adding any fertilizers or off-site organic materials during this time.) If the percent organic matter remains relatively constant over time, and if nutrient levels are not excessive over the same time period, you can determine the sustainable percent organic matter for that soil system.
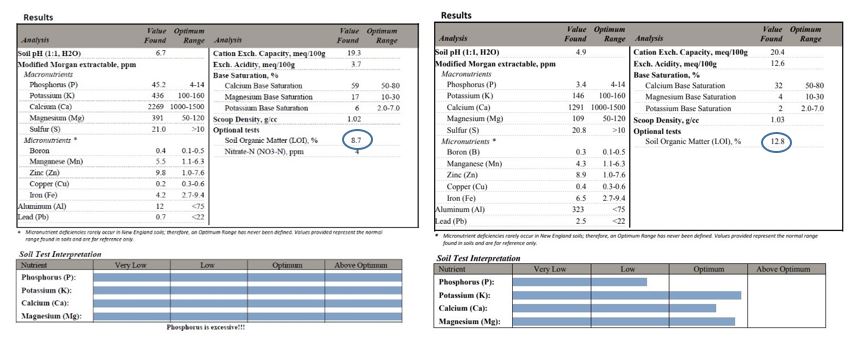
Compare these two soil tests below: one has excessive macronutrients, the other does not (Figure 3). However, the second soil has a substantially higher percent organic matter.[1] What this indicates is that the organic matter in the second system breaks down at a slower rate, so that the nutrient load does not exceed optimal levels. The first soil has excessively high nutrients (which could have been from fertilizer addition, not just organic material). The point is that nutrient load is the best indicator of whether the system has too much organic matter to be sustainable.
Figure 3a-b. Two soil tests, one with excessive nutrients and 8.7% organic matter (a), the other with optimal nutrients and 12.8% organic matter (b). Test results from University of Massachusetts soil testing lab.
[1] This particular soil is from the landscape of the first author. The soil there has supported centuries of white oak growth, returning significant levels of slow-to-decompose oak leaves to the soil. This is undoubtedly the source of the relatively high organic matter, which contributes available nutrients at a sustained pace.
- Excessive nutrient levels. Based on decades of research on both crop and ornamental species, scientists have determined optimal levels of macro- and micronutrients required for normal plant growth. While nutrient deficiencies can be common in intensive agricultural production, nutrient toxicities are unusual and as a result have received little research attention until recently. The relatively young field of urban soils research has begun to document the occurrence of nutrient toxicities as a result of improper fertilizer use and/or organic material additions. Phosphate toxicity is perhaps the best example of this phenomenon, but excessive levels of other nutrients such as calcium and magnesium are also commonly seen in garden and landscape soil tests, possibly due to underlying soil conditions or the improper use of gypsum and Epsom salts, respectively.
Myth 2: “Use acidic and alkaline amendments to change soil pH”
The pH of any soil is determined by soil type, the plants naturally growing in the region, and climate factors such as rainfall. Forests tend to predominate in wet climates and nutrients are tied up in the organic matter of the forest system, which efficiently recycles those minerals so that they are not lost from the underlying soils. Organic matter supplies soluble organic acids, which lower soil pH (Richie and Dolling, 1985); therefore, regions where forests naturally predominate tend to have more acidic soils. In arid regions, evaporation exceeds rainfall and in these soils salts accumulate in the upper layers of soil. While nutrients can be abundant, salinity levels may be high and may impair plant growth. The abundant levels of positively charged nutrients such as potassium, sodium, calcium, and magnesium bind with acidic components and create more alkaline soil conditions.
Measuring soil pH can be done with a soil test, with a portable meter, or with pH strips (Figure 4). However, it is also helpful to know nutrient content of the soil, and this can only be found through soil testing by a university or commercial lab (which will also report the pH). Soil test kits sold to homeowners are notoriously inaccurate and pH can be seriously under- or over-estimated by them (Faber et al., 2007).
Figure 4. Readily available test strips can be used to measure soil pH with varying degrees of accuracy. Photo courtesy of Wikimedia.
Because many environmental factors determine soil characteristics, including pH, it is difficult if not impossible to modify underlying soil chemistry in any permanent way. As discussed earlier, organic matter applied to soil will disappear over time, as will its effects on soil pH. Additions of lime (which contains calcium) can raise soil pH, while elemental sulfur and ammonium products can acidify the soil. But this is a temporary measure (especially in sandy soils) and potentially creates nutrient toxicities if those elements are already sufficiently available in the soil. Furthermore, a landscape soil system is so vast that a great deal of amendment would need to be added to have a measurable effect on underlying pH. Finally, the addition of such amendments requires significant tillage, which we have recommended against elsewhere (Chalker-Scott and Downer, 2019).
Myth 3: “Garden and landscape plants require routine fertilization”
Organic, inorganic, slow release, soluble, granular, complete or micronutrient - there are many fertilizer options available for gardeners at garden centers. Gardeners generally apply fertilizers on a regular basis for “maintenance,” often in the complete absence of any actual plant nutrient deficiency. Unfortunately, most gardeners do not want to take the time to pursue soil analysis and determine exactly what minerals are deficient - so they just apply a complete fertilizer (which is simply a balanced NPK formulation) on a routine basis. Unnecessary fertilizer application has negative consequences on plant and soil health, resulting in changes to vegetation structure, food availability, and other phenological properties in ways that can harm wildlife populations such as birds (Lepcyzk et al., 2004).
Some fertilizers offer plant specific formulations, such as rose food, citrus food (Figure 5), azalea fertilizer (or more generically acid-loving plant fertilizer). However, there is no published research that prescriptive fertilizers will enhance growth of the intended plant any better than generic fertilizer products. In fact, just fertilizing with nitrogen – the most commonly deficient nutrient - may solve many perceived deficiencies in garden plants.

Figure 5. Specialty fertilizers are heavily marketed for specific crops, but there is no published evidence they are any different from generic fertilizers. Photo by A.J. Downer.
Frequent fertilizer application is often used in an attempt to produce faster growth – but fertilizers are not growth regulators and do not cause plants to grow faster than normal. But lusher growth of landscape plants, due to uptake of nutrients in excess of what’s needed for normal growth, can predispose them to some diseases, such as powdery mildew. Nor does adding nutrients to trees and other woody plants improve their resistance to insect pests (Herms, 2002). Plants that suffer from poor drainage, insufficient light, and other environmental factors that limit plant growth are not assisted by increased fertilizer use. A sustainable approach to landscaping (Figure 6, which includes judicious use of woody mulch (Chalker-Scott, 2015,) reduces use of fertilizers (Hartin et al., 2014).
Figure 6. An arid climate landscape. Photo by A.J. Downer.
Before any application of fertilizer, soils should be analyzed by a university or commercial soil lab to determine actual mineral contents (Figure 3). While plant appearance can indicate foliar nutrient deficiencies, this does not necessarily mean that soils are likewise limited. Excessive levels of some soil nutrients can interfere with availability and uptake of other nutrients; the discussion of excessive phosphorus use later in this article is a good example. Poor root health, due to improper planting (Chalker-Scott and Downer, 2019, 2018) also restricts nutrient uptake, resulting in foliar deficiency symptoms. Additionally, plants that suffer from root rot can show nutrient deficiency symptoms, as their roots are not able to absorb the necessary nutrients. Finally, some plants may not be well adapted to the site or climate in which they are growing (which may manifest as poor root health).
Routine fertilization is a problem for plants, soils, and the surrounding environment. In arid climate soils, inorganic fertilizers add more salts to already salty soils, thus making physiological drought stress from salinity more of a problem for plants. In wetter climates, excessive nutrients from unnecessary fertilization leach into streams and other aquatic systems, impairing water quality. Many landscape plants require little or no fertilization to perform adequately in landscapes (Figure 7). Fertilizing plants that don’t require it is expensive, unnecessary, and damaging to plant, soil and environmental health.

Figure 7: These Salvia plants did not respond to routine fertilization with a soluble complete fertilizer. The clay soil in which they are growing already contained sufficient nutrients. Photo by A.J. Downer.
Myth 4: “Add phosphate fertilizer at transplant to enhance root growth”
Phosphorus, the “P” in the NPK label (Figure 8) found on fertilizers, is a macronutrient required for the synthesis of essential macromolecules including nucleic acids (like DNA) and energy transfer compounds (like ATP). It is one of the “big three” nutrients (along with nitrogen and potassium) that is often deficient in intensively managed agricultural fields. Phosphate is the major, and sometimes only, component of so-called “transplant” fertilizers, which are widely advertised as root-growth stimulators for new trees and shrubs. But residential and other nonagricultural soils are rarely deficient in phosphate. Additional phosphate, applied routinely without knowledge of existing soil phosphate concentrations, can lead to phosphate toxicity and associated mineral imbalances (Chalker-Scott and Olmsted, 2009).

Figure 8. Bone meal fertilizer recommended for transplanting. Note high P level in formulation (3-15-0). Photo by Linda Chalker-Scott
Phosphorus toxicity can often be visualized as interveinal chlorosis of leaves (Figure 9). The chlorosis is due to foliar deficiencies of iron and/or manganese linked directly to excess soil phosphorus. Research has documented this problem over the last several decades (e.g., Delmas et al., 1957; Fox and Warner, 1971), yet this information does not make its way into Extension recommendations for home gardeners.
Figure 9. Interveinal chlorosis. Photo by Linda Chalker-Scott.
When plants are exposed to increased levels of phosphorus, iron phosphates are precipitated out of solution both in the soil and within plant tissues. This form of iron cannot be taken up by roots, nor can it function in its biochemical role as an enzyme cofactor. The lack of functional iron in leaves causes interveinal chlorosis (Figure 9). Chalker-Scott and Olmsted (2009) not only found an increase in Rhododendron leaf chlorosis, but also saw oedema, root damage, and death with increasing soil phosphorus levels (Figure 10).
Figure 10. Leaf chlorosis as a result of increasing phosphate fertilizer (C=control, no fertilizer; ½X = half of recommended application; 1X = recommended application; 2X = twice recommended application). 1= Normal (green leaves), 2= Light chlorosis in young leaves, 3= Moderate interveinal chlorosis, 4= Severe chlorosis, young leaves white (from Chalker-Scott and Olmsted, 2009).
In addition to these induced nutrient deficiencies, excessive phosphorus also inhibits mycorrhizae, whose presence is crucial to the health of most ornamental plants (Chalker-Scott, 2017). Phosphorus is also the principal nutrient responsible for aquatic eutrophication and the link between the use of phosphorus-containing fertilizers and fish kill (Figure 11) is well known (Oliveira and Machado, 2013). Before any phosphorus-containing fertilizers are added to garden and landscape soils, a soil test should be performed to verify the need. The potential for soil, plant, and environmental damage is too great to ignore.

Figure 11. Eutrophication results from excessive levels of phosphorus, which stimulates algal growth and eventually algal death and decay. The decay process by microbes depletes oxygen in the water, leading to the death of fish and other aquatic life. Photo courtesy of Colourbox.
Myth 5: “Add Epsom salt to garden and landscape soils as a general fertilizer”
Epsom salt (Figure 12), also called magnesium sulfate, contains two plant macronutrients: magnesium and sulfur. Sulfur is a part of certain amino acids, while magnesium plays a role as an enzyme cofactor – a chemical that can assist in chemical reactions by accepting or donating an electron. While both of these are essential elements, neither is needed in excess. Yet many garden and landscape soils, as mentioned earlier, are improperly amended with Epsom salt.

Figure 12. Epsom salt appeals to gardeners, though its use is rarely necessary. Photo courtesy of Needpix.com.
The internet is full of unsubstantiated claims for Epsom salt application to gardens and landscape, including:
- Cures blossom end rot;
- Deters pests;
- Helps seeds germinate;
- Increases chlorophyll production;
- Produces more flowers; and
- Improves bloom quantity and quality of roses (Figure 13).
Figure 13: Despite the assertion of Rosarians, there is no published evidence supporting use of Epsom salt in rose culture. Photo by A.J. Downer.
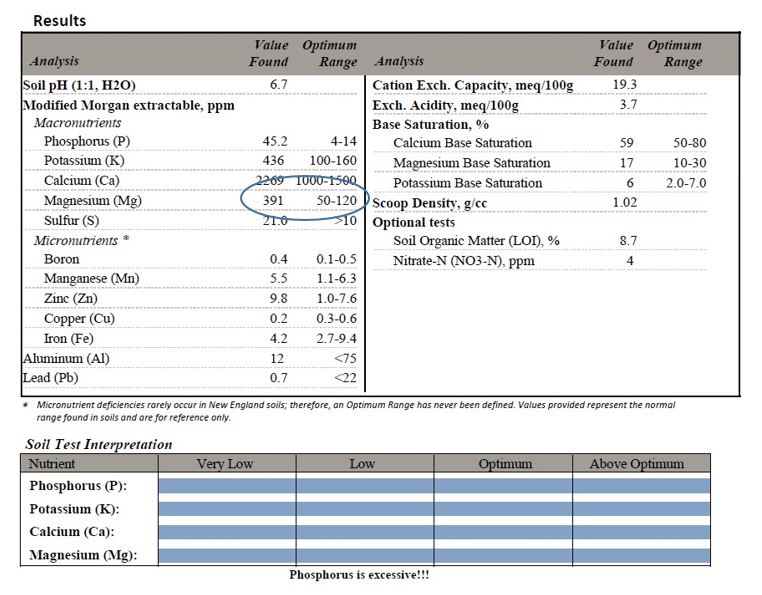
There are no documented benefits to support these claims (Chalker-Scott and Guggenheim, 2018), and they should be ignored. Instead, a soil test should be performed at an appropriate soil testing lab to determine if there is magnesium deficiency (Figure 14). If a deficiency is present, follow soil lab guidelines for appropriate additions. If there is no deficiency, do not add this chemical to your garden or landscape.
Figure 14. In this soil, magnesium (391 ppm) far exceeds that which is recommended (50-120 ppm). Nothing containing magnesium should be added. Test results from University of Massachusetts soil testing lab.
Do not rely on leaf symptoms that appear to indicate a soil magnesium deficiency (Figure 15). Chlorosis, or leaf yellowing, is a common result of many environmental stresses, including drought, cold, and nutrient imbalances. Adding magnesium when there is no soil deficiency can interfere with calcium uptake and possibly other nutrients (Chalker Scott and Guggenheim, 2018a). Only a soil test can identify a deficiency of magnesium or any other nutrient.

Figure 15. Magnesium deficiency presents as chlorosis, but many other factors can also cause leaf yellowing. Photo courtesy of Wikimedia.
Why does this myth persist, especially in the complete absence of any scientific literature? Speculation suggests that there is an emotional appeal behind the name “Epsom salt,” conjuring images of soothing spa treatments. It is doubtful whether marketing this product as simply magnesium sulfate would generate the enthusiasm found for Epsom salt.
Myth 6: “Gypsum will create better soil by modifying structure, texture, and pH”
Gypsum is calcium sulfate and has a long and confusing history of use in gardening. Retail packaging often asserts that gypsum will modify soil texture, improve soil structure, increase water penetration and drainage, loosen compacted soils, and neutralize salts (Figure 16). Although not suggested on most labels, many gardeners mistakenly believe gypsum reduces soil pH due to its sulfur content (sulfur content often is a marketing point for gypsum products). Regardless of its perceived acidification ability, adding gypsum does not change soil pH (Chalker Scott and Guggenheim, 2018); soil pH is governed by climate and environmental factors as discussed earlier.

Figure 16: This product claims to “loosen hard soil” – a misleading statement. Photo by A.J. Downer.
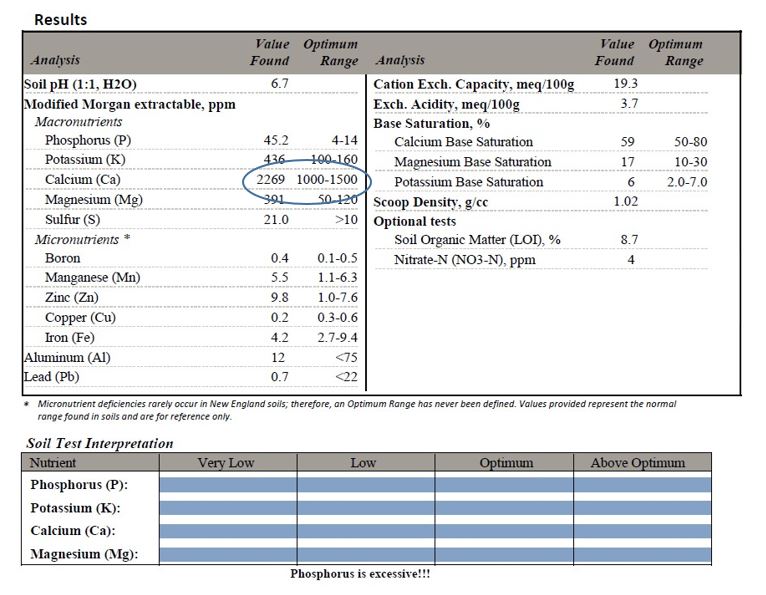
Before gypsum is added, it is crucial to determine if a calcium deficiency is present in the soil (Figure 17). If it is not deficient, gypsum should not be added. Additions will simply create nutrient imbalances which are difficult to correct.
Figure 17. In this soil, calcium (2269 ppm) far exceeds that which is recommended (1000-1500 ppm). Nothing containing calcium should be added, including gypsum. Test results from University of Massachusetts soil testing lab.
If there is a deficiency in calcium as confirmed by a soil test, the addition of gypsum will address that deficiency. Past this, the other claimed benefits are dependent on soil characteristics. Gypsum has documented benefits as in the following cases:
- Sodic soils. Sodic soils contain sodium and will not allow water to easily enter or drain from them. Adding gypsum to these soils will allow soil particles to bind together or flocculate. This is a chemical reactions where calcium (which has two positive charges) replaces sodium (with one positive charge) on clay particles (which have a negative charge). Sodic clay particles are covered with a “wool” of sodium ions which can only bind to one clay particle. Thus, the clay particles cannot bind to one another. Calcium has two positive charges, meaning that it can bridge two clay particles and pull them together. However, sodic soils are extremely uncommon in most gardens.
- Heavy clay soils. Many gardeners claim they have a clay soil simply because it doesn’t drain well. Most of the time this is due to soil compaction or textural discontinuities (Chalker-Scott and Downer, 2019). A heavy clay soil is one that contains >40% clay, which must be verified by laboratory analysis. (An approximation can be made by a method described elsewhere [Chalker-Scott and Downer, 2019].) If a soil contains high clay content but is low in calcium, gypsum can increase water infiltration and drainage (Chalker-Scott and Guggenheim, 2018) through flocculation as described above. This is especially important in poorly drained clay soils, where additions of gypsum can control root rot diseases caused by Phytophthora (Messenger 2000).
The miracles of improved drainage, loosened compacted soil, enhanced soil structure, etc. are not usually achieved with gypsum application in soils other than heavily sodic soils. To achieve the claims on gypsum product labels in non-sodic clay soils, mulching with fresh wood chips is far more effective (Chalker-Scott, 2015).
What to do instead: Action items for gardeners and landscape professionals
- Recognize that garden and landscape soils are often dissimilar to whatever soil was there before development. In other words, your soil has been modified through human activity so it bears little resemblance to the original, native soil reported in soil maps.
- Before adding any minerals, have a baseline soil test performed to determine soil pH, nutrient deficiencies and toxicities, and percent organic matter. Repeated annual testing will allow you to understand the mineral characteristics of your soil and its nutrient load.
- Understand that you cannot permanently change the pH of your soil. Instead, select plants that will tolerate your soil pH and manage the soil sustainably.
- Do not use “home remedy” fertilizers. If you have a documented nutrient deficiency, purchase the appropriate fertilizer and add it according to recommendations from your soil test
LITERATURE CITED
Chalker-Scott, L. (2017). A gardener's primer to mycorrhizae: understanding how they work and learning how to protect them. WSU Extension Fact Sheet FS269E. Pullman, Washington, USA. https://pubs.extension.wsu.edu/a-gardeners-primer-to-mycorrhizae-understanding-how-they-work-and-learning-how-to-protect-them-home-garden-series
Chalker-Scott, L. (2015). Using arborist wood chips as a landscape mulch. WSU Extension Fact Sheet FS160E. https://www.researchgate.net/publication/315662938_Using_arborist_wood_chips_as_a_landscape_mulch_WSU_Extension_Fact_Sheet_FS160E.
Chalker-Scott, L., and Downer, A.J. (2019). Soil myth busting for Extension educators: reviewing the literature on soil structure and functionality. Journal of the NACAA 12(2). https://www.nacaa.com/journal/post_editor.php?article_id=1024
Chalker-Scott, L., and Downer, A.J. (2018). Garden myth busting for Extension educators: reviewing the literature on landscape trees. Journal of the NACAA 11(2). https://www.nacaa.com/journal/post_editor.php?article_id=885
Chalker-Scott, L., and Guggenheim, R. (2018). Epsom salt use in home gardens and landscapes. WSU Fact Sheet FS308E. Pullman, Washington, USA. https://www.researchgate.net/publication/333516299_EPSOM_SALT_USE_IN_HOME_GARDENS_AND_LANDSCAPES
Chalker-Scott, L., and Guggenheim, R. (2018). Gypsum use in home gardens and landscapes. WSU Fact Sheet FS307E. Pullman, Washington, USA. https://www.researchgate.net/publication/333516307_GYPSUM_USE_IN_HOME_GARDENS_AND_LANDSCAPES
Chalker-Scott, L., and Olmsted, S. (2009). Iron deficiency in Rhododendron is due to excess soil phosphorus, pp. 356-367. In: G.W. Watson, L. Costello, B. Scharenbroch and E. Gilman (eds.), The Landscape Below Ground III: Proceedings of an International Workshop on Tree Root Development in Urban Soils. International Society of Arboriculture, The Morton Arboretum, Lisle, IL.
Delmas, J., Bats, J., and Remy, P. (1957). Manganese deficiency induced by excess phosphorus in peach grown in nutrient culture. Compte Rendu de l’Academie des Sciences 244:1971-1974.
Erickson, J.E., Volin, J.C., Park, D.M., Cisar, J.L., and Snyder, G.H. (2005). Phosphorus and potassium leaching under contrasting residential landscape models established on a sandy soil. Crop Science 45(2):546-552. https://www.researchgate.net/publication/237485882_Phosphorus_and_Potassium_Leaching_under_Contrasting_Residential_Landscape_Models_Established_on_a_Sandy_Soil
Faber, B.A., Downer, A.J., Holstege, D., and Mochizuki. M.J. (2007). Accuracy varies for commercially-available soil test kits analyzing nitrate nitrogen, phosphorus, potassium and pH. HortTechnology 17:358-362. https://www.researchgate.net/publication/279468671_Accuracy_Varies_for_Commercially_Available_Soil_Test_Kits_Analyzing_Nitrate-Nitrogen_Phosphorus_Potassium_and_pH
Fox, R.L., and Warner, R.M. (1971). Excess phosphate and micronutrient deficiency in macademia. Hawaii Farm Science 20(4):1-4.
Hartin, J., Geisel, P., Harivandi, A., and Elkins, R. (2014). Sustainable landscaping in California—How to conserve resources and beautify your home landscape. ANR publication 8504. University of California http://anrcatalog.ucanr.edu/
Herms, D.A. (2002). Effects of fertilization on insect resistance of woody ornamental plants: reassessing an entrenched paradigm. Environmental Entomology 31(6):923-933.
Lepcyzyk, C.A., Mertig, A.G., and Liu, J. (2004). Assessing landwoner activities related to birds across rural-to-urban landscapes. Environmental Management 33(1):110-125.
Messenger, B.J., Menge, J.A., and Pond, E. (2000). Effects of gypsum soil amendments on avocado growth, soil drainage and resistance to Phytophthora cinnamomi. Plant Disease 84: 612-616. https://www.semanticscholar.org/paper/Effects-of-Gypsum-Soil-Amendments-on-Avocado-Soil-Messenger-Menge/3cd02316312913d1ff08e95cb5949ffe34e7915b
Oliveira, M., and Machado, A.V. (2013). The role of phosphorus on eutrophication: a historical review and future perspectives, Environmental Technology Reviews 2(1):117-127. 10.1080/21622515.2013.861877
Richie, G.S.P., and Dolling, P.J. (1985). The role of organic matter in soil acidification. Australian Journal of Soil Research 23:569-576. https://www.researchgate.net/publication/248884087_The_Role_of_Organic_Matter_in_Soil_Acidification
Warner, L.A., Lamm, A.J., and Chaudhary, A.K. (2018). Florida residents’ perceived role in protecting water quantity and quality through landscape practices. Landscape and Urban Planning. 171:1-6. https://doi.org/10.1016/j.landurbplan.2017.11.007