Journal of the NACAA
ISSN 2158-9429
Volume 10, Issue 2 - December, 2017
Using Microbial Inoculants to Improve Fermentation and Nutritive Value of Annual Ryegrass Baleage
- Lemus, R. , Extension Forage Specialist, Associate Extesion/Research Professor, Mississippi State University Extension Service
Rivera, J.D., Associate Research/Extension Professor, Mississippi State University
White. J.A., Forage Variety Testing Manager, Mississippi State University
ABSTRACT
Baleage, or haylage, is forage that is baled and wrapped soon after cutting at high moisture content (40 to 60%). The objective of the study was to assess the effectiveness of different microbial inoculants and application rates on ensiling period, fermentation, and forage quality of annual ryegrass baleage. ‘Marshall’ annual ryegrass was harvested from the cool-season forage variety trial in May of 2013 and left to air dry in the field for 24 and 48 h before ensiling. Forage was treated with seven microbial inoculants containing homolactic and heterolactic bacteria at a 1X and 2X rate and ensiled for 30, 45 and 60 d. At the end of ensiling period, pH, ˚Brix, and forage nutritive value were measured. There was a microbial inoculant effect for pH, °Brix, and nutritive value. The Biotal Buchneri 500 had the lowest pH and the greatest ˚Brix concentration. Inoculant rate were significantly different for crude protein (CP), acid detergent fiber (ADF), neutral detergent fiber (NDF), and in vitro true dry matter digestibility (IVTDMD). Ensiling dates caused a decrease in pH, ˚Brix levels, and dry matter when increased from 30 to 60 days. Crude protein was very stable and IVTDMD were fairly stable while ADF and NDF levels increased. Most of the effects were due to ensiling period and the treatment interaction, especially for pH. These inoculants showed major response in stimulating rapid fermentation and inhibiting spoilage.
Key Words: Annual ryegrass, baleage, haylage, microbial inoculants, ensiling, fermentation, forage quality.
Introduction
Ensiling higher moisture hay is an alternative forage storage method to making dry hay that requires relatively less drying time for successful preservation. Haylage, grass silage, or baleage are terms given to hay that has been ensiled soon after cutting, minimizing the risk of loss through rain and drying time. Baleage production has increased in popularity in the southern USA where high rainfall and humidity limit successful hay production (McCormick, 2013). The wrapping process creates an environment favorable to anaerobic fermentation of existing carbohydrates in the grass, thereby preserving the harvested product. It has potential to be a major feed source in livestock operations.
Baleage harvest is handled in the same manner as it would be for dry hay. A decision can be made for dry hay or baleage based on weather conditions at the time the hay is cut. The amount of moisture content in the baleage is important to the fermentation process. As a general recommendation, the moisture content must be between 40 and 60% (Allen et al., 2011). When moisture levels are below 30% there is not enough moisture to properly ferment the baleage. At moisture content above 60%, the excess moisture can cause leakage in the wrapping and resulting spoilage due to improper sealing and fermentation. Additionally, preservation above 60% moisture may lead to clostridial bacteria formation instead of the lactobacillus, which could potentially be fatal or harmful to livestock (Collins and Owens, 2003). However, other environmental conditions such as wilting time, humidity, and temperature may require the moisture content to average 50% to be in a more specific range for economic benefits.
In general, the pH level of the forage that is pre-ensiled is between 5.5 and 6.0. During the ensiling process, the pH level will drop due to acid production, with the final pH likely being between 3.7 and 4.7, depending on the forage type, nonstructural carbohydrate content, moisture level and bacterial inoculant. McCormick et al. (1998) have indicated that the pH of ensiled grasses in the southern USA can range from 4.1 to 5.2. Baleage is typically more difficult to ensile because grasses usually tend to have a higher buffering capacity (resistance to pH drop) and lower levels of water soluble carbohydrates (WSC) and lactic acid producing bacteria (LAB) (Andesogan, 2008). High levels of LAB bacteria are needed to aid in dropping the pH to achieve a more efficient fermentative stability, and prevent the growth of undesirable bacteria such as enterobacteria and clostridia. High levels of undersirable bacteria during the fermentation process can increase dry matter and protein degradation (Adesogan, 2008). To avoid these types of losses, the use of preservatives could speed up the fermentation process, especially at less than ideal moisture content. There are many different types of microbial inoculants with conflicting data regarding their efficiency.
Microbial inoculants are initially inactive bacterial strains that when added to baleage become active and can aid in breaking down plant sugars. Some of the benefits from inoculants include more efficient fermentation, reduced temperatures in the baleage, reduced dry matter and energy losses, reduced protein solubilization, longer forage shelf life and improved animal performance (Buckmaster and Lundmark, 1992). Microbial inoculants could be used as aids to stimulate or ensure rapid fermentation and as inhibitors of aerobic conditions that could create spoilage. Some of these inoculants have also been designed to specifically improve the aerobic stability at post-ensiling. Baleage inoculants are biological products that contain a source of live, viable bacteria and sometimes combine different species of fermentative bacteria (Kung et al., 2003 a,b). The most common types of bacteria that are found in microbial inoculants are those known as homolactic, heterolactic and propionic bacteria. Some of most common homolactic bacteria include species such as Lactobacillus bucheneri, Enterococcus faecium, and Peidococcus pentosaceus (Kleinschmit and Kung, 2006). These homolactic bacteria can improve the initial fermentation process by increasing the production of lactic acid, increasing the efficiency of fermentation and limiting the production of secondary end products such as enterobacteria and clostridia (Ranjit and Kung, 2000; Driehuis et al., 2001; Ranjit et al., 2002; Adesogan et al., 2003). However, the use of homolactic bacterial inoculants are not always very effective in improving aerobic stability and shelf life of the baleage because lactic acid alone is a poor antifungal agent (Weinberg and Muck, 1996). On the other hand, Arriola et al. (2011) indicated that forages ensiled with the obligate heterofermentative Lactobacillus buchneri typically has improved aerobic stability. An example of a heterolactic acid bacterium is Lactobacillus plantarum that in addition to the formation of lactic acid, yields carbon dioxide and ethanol. Propionibacterium shermanii (PAB) is a type of propionic acid bacteria used in ensiling process because it produces lactic acid and propionic acid (Krooneman et al., 2002). This heterofermentative bacteria is used to lengthen the aerobic stability of the baleage in the feed out phase (Weinberg et al., 1999). It is always important to make sure the LAB dominate the fermentation process resulting in lower pH, less acetic and propionic acid, and less ethanol in the preserved forage (Moshtaghi and Wittenberg, 1999). To ensure that consistent, positive results are achieved, faster-acting bacteria such as Pediococcus pentosaceus, Pediococcus acidilacti, Enterococcus faecium, and Lactobacillus acidophilus have been included with L. plantarum (Weinberg and Muck, 1996).
The epiphytic LAB ferments WSC into lactic acid and other short chain volatile fatty acids (Filya et al, 2004). However, application of L. buchneri alone can increase DM losses slightly (Ranjit and Kung, 2000). Therefore, L. buchneri is often combined with homofermentative or facultative heterofermentative bacteria in inoculants to constrain DM losses and improve aerobic stability (Driehuis et al., 2001; Adesogan et al., 2004; Arriola et al., 2011). Homolactic bacteria have recently been included in L. buchneri inoculants to enhance the anaerobic fermentation phase and thereby, prevent increases in pH and DM losses resulting from inoculation with L. buchneri alone (Kleinschmit and Kung, 2006). Adesogan et al. (2006) compared the efficacy of a mixture (1.1 x 1011, colony forming unit or cfu/g) of L. plantarum, L. buchneri and E. faecium or a mixture of P. pentosaceus (1 × 105 cfu/g) and L. buchneri (4 × 105 cfu/g). The inoculants were applied as recommended or at twice the recommended rate. All inoculants reduced lactic to acetic acid ratios and yeast counts and increased aerobic stability, but inoculant type and application rate did not affect these measures. In almost all cases, aerobic stability was increased by inoculation, but pH or DM losses were not increased, indicating that the inoculants did not adversely affect the fermentation.
A wide variety of additives are marketed to improve baleage quality. In the southern USA, the main baleage additive is bacterial inoculant. Converting annual ryegrass from the traditional hay system into baleage might help conventional livestock producers to maintain better quality forage and reduce supplementation. The use of microbial inoculants have been shown to improve fermentation or aerobic stability of baleage under certain harvest and storage conditions. This type of additive supplements the natural lactic acid bacteria present on the forage to help guarantee a fast and efficient silage fermentation. Silage inoculants have improved in the last decade and data on the effectiveness of additives in annual ryegrass baleage is very limited and warrants further investigation. The objective of the study was to assess the effectiveness of different microbial inoculants and application rates on ensiling period, fermentation, and forage quality of annual ryegrass baleage.
Material and Methods
Evaluating the effect of microbial inoculants on baleage stability and nutritional value using field-scale wrapped bales is challenging because of the difficulty of producing homogeneous baleage for treatment evaluation (Romero et al., 2017). Alternatively, small sealed laboratory bags provide a more complete control of the ensiling conditions and process and have been widely used when multiple microbial inoculant treatments are evaluated. The study was conducted at the Henry H. Leveck Animal Research Farm at Mississippi State University (33º 25’ 18” N, 88º 47’ 30” W). The soil type is a Savannah fine sandy loam (Fine-loamy, siliceous, semiactive, thermic Typic Fragiudults). 'Marshall' annual ryegrass was harvested from the cool-season forage variety trial in May of 2013 in Starkville, MS. Annual ryegrass was fertilized with 50 pounds of nitrogen per acre uinsg urea-ammoniun sulfate (33-0-0) when plants reached three inches after emergence and after each harvest in the winter and spring. Phosphorus and potassium applications were applied based on soil test recommendations.
The experimental design was a completely randomized block with factorial arrangement of treatments replicated three times. A 4-wk regrowth of annual ryegrass was cut in with a sickle bar mower when grass was 12 inches tall and let to wilt in the field to simulate typical environmental conditions that a producer will encounter when making baleage. Three random forage samples were collected at 0, 24 and 48 h after harvest for dry matter determination. The dry matter content at 0, 24 and 48 h was 18.6, 26.0 and 40.7%, respectively. Forage samples were ensiled at two post-harvest ensiling times, 24 and 48 h. Seven commercial microbial inoculants were used in the study and three other treatments included a check (control), check + water, and a sugar solution (Table 1). The microbial inoculants were applied at 1X and 2X the recommended rate. Microbial inoculant solutions were prepared by mixing 1 gram of product into 1000 ml of deionized water and using 1 ml of the solution to treat one pound of baleage. Annual ryegrass samples were cut to approximately 2.54 cm (1 inch) in length and then approximately one pound of forage was treated with 3.5 ml of inoculant treatment solution and mixed thoroughly. Annual ryegrass samples were placed in freezer bags, vacuum sealed, placed in three separate 20-gal Rubbermaid black plastic containers for each ensiling period with a tight seal and allowed to ferment in the dark for an ensiling period of 30, 45 and 60 days. Containers were placed in a dark room in the laboratory with temperature range of 77 to 80 ˚F and 70 to 72% relative humidity. After each ensiling period, samples were removed from the vacuum sealed bags and checked for pH using a Fisher Accumet AE150 digital pH meter. Degrees Brix (°Brix) level was also determined using a PDX-1 Vee Gee digital refractometer. Samples were weighed and placed in a forced-air drying oven at 150 ˚F for a period of 72 h until a constant weight was obtained and ground to pass a 2-mm screen. Ground samples were used to determine nutritive value [crude protein (CP), acid detergent fiber (ADF), neutral detergent fiber (NDF), and in vitro dry matter digestibility at 48 hours (IVTDMD)] using a Foss 2500 NIR instrument and the 2012 haylage equation developed by the NIRS Forage and Feed Testing Consortium (Hillsboro, WI). The Foss 2500 NIR Instrument is an instrument certified by the National Forage Testing Association. Data was analyzed with PROC GLIMMIX in SAS and means separated using the Least Significant Differences (LSD) at α = 0.05.
| Inoculant Treatments | Bacteria Content | Rate (mg/L)* | |
| 1X | 2X | ||
| Check 1 (-) (CHK) | No inoculant | 0 | 0 |
| Check 2 (+) (CHK-S) | Sugar | 107 | 214 |
| Check 3 (-) (CHK-W) | Water | 0 | 0 |
| Biotal Buchneri 500 (BB-500) | Lactobacillus buchneri (NCIMB 40788) and Peidococcus pentosaceus (NCIMB 12455) (500,000 cfu/g) | 214 | 428 |
| Biotal Buchneri 40788 (BB-40788) | Lactobacillus buchneri (NCIMB 40788) (400,000 cfu/g) | 214 | 428 |
| Biotal Hay (BH) | Peidococcus pentosaceus (NCIMB 12455) (500,000 cfu/g) | 214 | 428 |
| Biotal Plus (BP-II) | Peidococcus pentosaceus (NCIMB 12455) and Propionicbacterium freudenreichii (R2453) (120,000 cfu/g) | 214 | 428 |
| Biotal Silage Inoculant II (BSI-II) | Lactobacillus plantarum (NCIMB 12422) and Peidococcus pentosaceus (NCIMB 12455) (100,000 cfu/g) | 214 | 428 |
| Ecosyl (ECO) | Lactobacillus plantarum strain MDD/1 (NCIMB40027) (100,000 cfu/g) | 54 | 108 |
| ProbioFerm (PBF) | Lactobacillus plantarum, Peidococcus acidilactic, and Enterococcus faecium (400,000 cfu/g) | 107 | 214 |
| *Amount of product in mg per 1000 ml (1L) of water to apply 3.5 ml of solution per sample. | |||
Results and Discussion
Fermentation analyses are often used to assess baleage quality. Different parameters that were measured as part of this annual ryegrass baleage study included pH, °Brix, crude protein (CP), acid detergent fiber (ADF), neutral detergent fiber (NDF), and in vitro true dry matter digestibility at 48 h (IVTDMD).
pH – Baleage pH can vary depending on the type of forage crop, dry matter content, stage of maturity of the forage crop, water soluble carbohydrates, phases on the ensiling process, buffering capacity, and proper anaerobic environment among other factors. In this study there was inoculant treatment effect in the fermentation process (P <0.0001). Inoculant treatments had a pH ranging from 3.92 to 4.83. BB-500 had the lowest pH while the checks had the greatest pH (Table 2). Microbial inoculant rate did not affect final fermentation pH (P = 0.1556). There was a post-harvest ensiling time x inoculant treatment effect (Table 3). Most of the interaction effects were observed with the check treatments. Differences among inoculant treatments within each post-harvest ensiling time indicated that BB-40788 and BB-500 had the lowest pH at 24 and 48 h post-harvest ensiling time, respectively (P <0.0001). Significant (P<0.0001) effect of ensiling period was observed where 30 d ensiling was compared to the 45 and 60 d period (4.11, 4.78, and 4.66, respectively). A significant inoculant treatment x ensiling period interaction (P <0.0001) was also observed for baleage pH. The BB-500 microbial inoculant had the lowest pH at the 30, 40, and 60 d ensiling period (Table 4). Interestingly, all treatments retained lower pH values at the 30 and 60 d ensiling period than the 45 d period. There was also a post-harvest ensiling time x ensiling period interaction (P <0.0001) in which samples ensiled at 24 h post-harvest retained lower pH values across the 30, 45, and 60 d ensiling period, respectively (Table 5). While there are target pH ranges, this measure is not an absolute, fail-safe parameter and is somewhat dependent on the mixture of acids produced, which can be influenced by the type of inoculant used.
| Nutritive Value | |||||||
| Inoculant Treatment | pH | ºBrix | CP | ADF | NDF | IVTDMD | |
| ------------------------------------ % DM ------------------------------------ | |||||||
| CHK | 4.83 | 18.28 | 13.49 | 31.40 | 46.82 | 83.83 | |
| CHK-S | 4.75 | 17.22 | 13.65 | 31.07 | 46.57 | 83.75 | |
| CHK-W | 4.66 | 17.78 | 13.54 | 30.99 | 46.36 | 83.82 | |
| BB-500 | 3.89 | 18.84 | 13.28 | 30.47 | 43.80 | 83.31 | |
| BB-40788 | 3.92 | 19.67 | 13.41 | 31.43 | 45.87 | 82.88 | |
| BH | 4.68 | 17.68 | 13.63 | 30.79 | 46.16 | 84.07 | |
| BP-II | 4.61 | 17.29 | 13.21 | 30.95 | 45.86 | 83.81 | |
| BSI-II | 4.58 | 18.41 | 13.53 | 30.84 | 46.44 | 83.79 | |
| ECO | 4.63 | 18.31 | 13.36 | 30.86 | 45.48 | 83.84 | |
| PBF | 4.64 | 17.75 | 13.18 | 30.88 | 45.71 | 83.54 | |
| LSD0.05 | 0.12 | 0.84 | 0.29 | 0.34 | 0.54 | 0.46 | |
Degrees Brix (°Brix) – ºBrix is a measure of sugar content in an aqueous solution. One-degree ºBrix is equivalent to 1 gram of sucrose in 100 grams of solution. There was a microbial inoculant treatment effect (P <0.0001) in which BB-40788 had the greatest ºBrix level compared to other treatments and the controls (Table 2). The 48 h post-harvest ensiling time had significant greater ºBrix level (P <0.0001) than the 24 h post-harvest treatment (Fig. 1). There was also an inoculant treatment x post-harvest ensiling time interaction (P <0.0001). The check and BB-40788 treatments had the greatest ˚Brix levels within the 24 and 48 h post-harvest ensiling time (Table 3). The 48 h post-harvest ensiling treatment had a consistent higher ˚Brix level within each microbial inoculant treatment than the 24 h post-harvest ensiling time. A significant (P <0.0001) decrease in ºBrix level was also observed from the 30 to the 60 d ensiling period (Fig. 3). ºBrix level decreased 15% from 30 to 60 d post-ensiling period. A significant post-harvest ensiling time x ensiling period was observed (P = 0.0001). It was observed that the 48 h post-harvest ensiling time had greater ºBrix level, but at the same time those levels decreased with longer ensiling periods (Table 5).
| Post-harvest Ensiling Time (h) | |||||||||||
| Treatment | 24 | 48 | LSD0.05 | 24 | 48 | LSD0.05 | 24 | 48 | LSD0.05 | ||
| ------------ pH ------------ | ------------ ºBrix (%) ------------ | ------------ CP (%) ------------ | |||||||||
| CHK | 4.62 | 5.04 | 0.32 | 14.89 | 21.67 | 1.33 | 13.33 | 13.66 | NS | ||
| CHK+S | 4.44 | 5.06 | 0.29 | 13.71 | 20.73 | 1.42 | 13.59 | 13.70 | MS | ||
| CHK+W | 4.34 | 4.98 | 0.26 | 14.17 | 21.39 | 1.09 | 13.72 | 13.37 | 0.60 | ||
| BB-500 | 3.90 | 3.88 | NS | 13.72 | 23.96 | 1.47 | 13.54 | 13.01 | 0.46 | ||
| BB-40788 | 3.88 | 3.96 | NS | 12.34 | 26.99 | 1.83 | 13.71 | 13.10 | NS | ||
| BH | 4.56 | 4.80 | NS | 13.31 | 22.06 | 1.57 | 13.80 | 13.46 | NS | ||
| BP-II | 4.65 | 4.57 | NS | 13.39 | 21.19 | 1.76 | 13.49 | 12.92 | 0.53 | ||
| BSI-II | 4.58 | 4.59 | NS | 14.09 | 22.73 | 1.11 | 13.70 | 13.36 | NS | ||
| ECO | 4.61 | 4.64 | NS | 14.19 | 22.42 | 1.36 | 13.83 | 12.90 | 0.61 | ||
| PBF | 4.52 | 4.75 | NS | 13.96 | 21.54 | 0.34 | 13.48 | 12.88 | NS | ||
| LSD0.05 | 0.23 | 0.31 | 0.91 | 1.78 | NS | 0.46 | |||||
| ------------ ADF (%) ------------ | ------------ NDF (%) ------------ | ------------ IVTDMD (%) ------------ | |||||||||
| CHK | 31.19 | 31.61 | NS | 46.96 | 46.68 | NS | 83.69 | 83.97 | NS | ||
| CHK+S | 30.81 | 31.33 | NS | 46.61 | 46.53 | NS | 83.77 | 83.72 | NS | ||
| CHK+W | 30.65 | 31.33 | NS | 46.25 | 46.47 | NS | 84.01 | 83.62 | NS | ||
| BB-500 | 30.06 | 30.89 | NS | 43.79 | 43.81 | NS | 83.23 | 83.40 | NS | ||
| BB-40788 | 32.44 | 30.42 | 1.07 | 48.46 | 43.28 | 1.77 | 81.94 | 83.82 | 0.93 | ||
| BH | 30.89 | 30.70 | NS | 46.40 | 45.91 | NS | 83.59 | 84.56 | 0.79 | ||
| BO-II | 30.57 | 31.32 | NS | 45.70 | 46.02 | NS | 83.61 | 84.00 | NS | ||
| BSI-II | 31.13 | 30.55 | NS | 46.89 | 46.00 | NS | 83.42 | 84.17 | NS | ||
| ECO | 30.45 | 31.27 | NS | 45.37 | 45.59 | NS | 83.89 | 83.85 | NS | ||
| PBF | 30.67 | 31.09 | NS | 46.10 | 45.33 | NS | 83.37 | 83.70 | NS | ||
| LSD0.05 | 1.06 | 0.68 | 1.38 | 1.25 | 0.93 | 0.58 | |||||
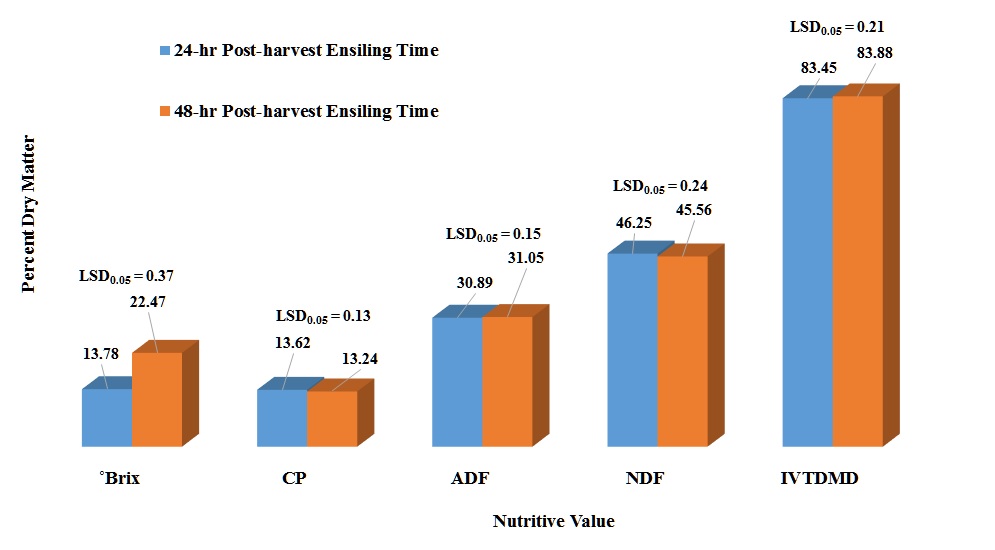
Figure 1. Influence of post-harvest ensiling time on ºBrix and nutritive value of annual ryegrass baleage.
Crude Protein (CP) – Statically, there were significant differences in the percent CP among the microbial treatments (P <0.0001) (Table 2). In reality, those differences are very small to have a justifiable economic impact in protein concentration. There was a significant post-harvest ensiling time (P <0.0001) for CP (Fig. 1) in which 24 h post-harvest ensiling time had a 3% CP increase. There was a rate effect (P = 0.0021) in CP concentration which the 2X had a slightly greater concentration (1.5 %), but not justifiable from the economic aspect (Fig. 2). There was a significant inoculant treatment x post-harvest ensiling time (P = 0.0012). Most of the differences were observed among microbial inoculant treatments within the 48 h post-harvest ensiling time which none of the treatments were better than any of the controls (Table 3). Comparison of CP percentages among post-harvest ensiling time for each microbial inoculant indicated that there was a decrease in CP from 24 to 48 h post-harvest ensiling time. This could be related to plant respiration before any anaerobic conditions were imposed. There were significant differences in CP for the three ensiling periods (P <0.0001), with the 45 d ensiling period having the lowest percent CP (Fig. 3). A significant post-harvest ensiling time x ensiling period was observed (P <0.0001). Percent CP declined from the 30 to 60 d ensiling period within the 24 hr post-harvest ensiling time, while the opposite trend was observed within the 48 h post-harvest ensiling time (Table 5).
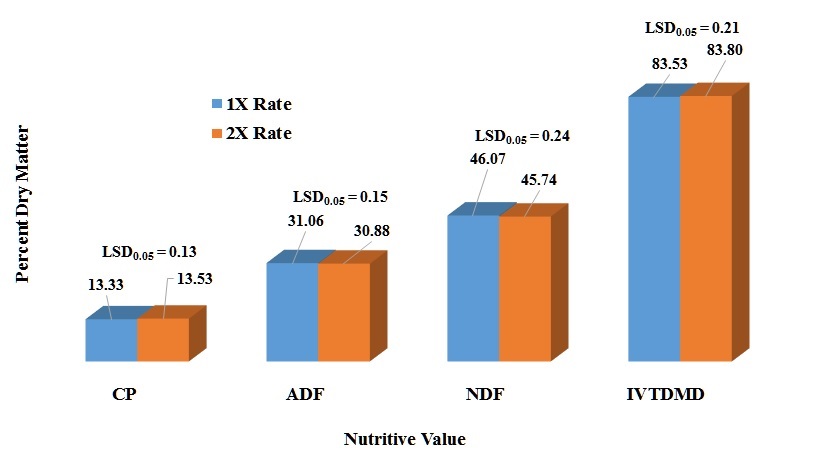
Figure 2. Influence of overall inoculant rate on nutritive value of annual ryegrass baleage.
Acid Detergent Fiber (ADF) – Acid detergent fiber is considered a measure of fiber content that include cellulose, lignin, silica, but not hemicellulose. It is usually used to measure digestibility. There were significant differences in the percent ADF among the microbial treatments (P <0.0001). BB-500 exhibited the smallest ADF concentration while BB-47088 had the greatest concentration (Table 2). There was a significant post-harvest ensiling time (P = 0.0332) for ADF (Fig. 1) in which 48 h post-harvest ensiling time had a slight increase in ADF concentration. There was a significant inoculant treatment x post-harvest ensiling time (P <0.0001). There were no significant differences among post-harvest ensiling times within treatments except for BB-40788 in which the 24 h post-harvest ensiling had higher ADF concentration (Table 3). Most of the differences were observed among inoculant treatments within a post-harvest ensiling time. BB-40788 and the check had higher ADF concentrations compared to the other inoculant treatments within the 24 and 48 h post-harvest ensiling time, respectively. There was a rate effect in ADF concentration (P <0.0219) in which the 1X had a slightly greater concentration, but not justifiable from the economic aspect (Fig. 2). A significant difference in ADF concentration was observed for the three ensiling periods (P <0.0001), with increase in ADF from 30 to 60 d ensiling (3.5%) (Fig. 3). A significant inoculant treatment x ensiling period interaction (P = 0.0040) was observed for ADF concentration. Acid detergent fiber concentrations increased with ensiling period within each inoculant treatment, but significant differences among inoculant treatments was only observed for the 30 d ensiling period (Table 4). This is an indication that fermentative stability due to microbial activity was limited to the first 30 days of the fermentation process. A significant interaction between post-harvest ensiling time and ensiling period (P <0.0001) indicated the 30 d ensiling period had lower ADF concentration at the 24 h post-harvest ensiling time, but ADF concentrations were greater at the 45 and 60 d ensiling periods, respectively (Table 5).
| Ensiling Period (d) | ||||
| Inoculant Treatment | 30 | 45 | 60 | LSD0.05 |
| ----------------------------------- pH ----------------------------------- | ||||
| CHK | 4.26 | 5.13 | 5.11 | 0.26 |
| CHK+S | 4.30 | 5.07 | 4.88 | 0.34 |
| CHK+W | 4.26 | 4.96 | 4.75 | 0.34 |
| BB-500 | 3.81 | 4.04 | 3.82 | 0.19 |
| BB-40788 | 3.74 | 4.08 | 3.94 | 0.23 |
| BH | 4.18 | 4.94 | 4.93 | 0.23 |
| BP-II | 4.24 | 4.86 | 4.72 | 0.31 |
| BSI-II | 4.15 | 4.95 | 4.64 | 0.19 |
| ECO | 4.11 | 4.91 | 4.86 | 0.23 |
| PBF | 4.09 | 4.89 | 4.94 | 0.27 |
| LSD0.05 | 0.14 | 0.28 | 0.32 | |
| ----------------------------------- ADF (%) ----------------------------------- | ||||
| CHK | 30.55 | 31.23 | 32.43 | 0.67 |
| CHK+S | 29.80 | 31.11 | 32.29 | 0.61 |
| CHK+W | 30.03 | 30.70 | 32.24 | 0.50 |
| BB-500 | 28.82 | 30.08 | 32.52 | 0.74 |
| BB-40788 | 30.14 | 31.12 | 33.04 | 1.20 |
| BH | 29.60 | 30.90 | 31.89 | 0.66 |
| BP-II | 29.65 | 30.78 | 32.42 | 0.97 |
| BSI-II | 29.52 | 30.99 | 32.00 | 0.60 |
| ECO | 29.81 | 30.99 | 31.79 | 0.82 |
| PBF | 29.59 | 30.89 | 32.15 | 0.55 |
| LSD0.05 | 0.80 | NS | NS | |
| ----------------------------------- NDF (%) ----------------------------------- | ||||
| CHK | 45.84 | 46.45 | 48.18 | 0.73 |
| CHK+S | 45.10 | 46.42 | 48.19 | 0.66 |
| CHK+W | 45.20 | 45.96 | 47.93 | 0.53 |
| BB-500 | 41.96 | 41.65 | 47.80 | 1.26 |
| BB-40788 | 44.40 | 44.53 | 48.69 | 2.65 |
| BH | 44.77 | 46.19 | 47.50 | 1.06 |
| BP-II | 44.50 | 45.15 | 47.92 | 1.42 |
| BSI-II | 45.01 | 46.55 | 47.77 | 0.89 |
| ECO | 44.03 | 45.32 | 47.09 | 0.96 |
| PBF | 43.98 | 45.48 | 47.68 | 0.70 |
| LSD0.05 | 1.15 | 1.30 | NS | |
Neutral Detergent Fiber (NDF) – Neutral detergent fiber is the most common measure of fibers in structural components of the pant cell such as cellulose, hemicellulose and lignin. A significant inoculant treatment effect was observed in this study (P <0.0001). BB-500 had the lowest NDF concentration while the check had the highest concentration (Table 2). A significant post-harvest ensiling time interaction (P <0.0001) was also observed in which the 24 h post-harvest had greater NDF concentration than the 48 h (Fig. 1). There was a significant inoculant treatment x post-harvest ensiling time (P = 0.0002). There were no significant differences among post-harvest ensiling times within treatments except for BB-40788 in which the 24 h post-harvest ensiling had higher NDF concentration (Table 3). Most of the differences were observed among inoculant treatments within a post-harvest ensiling time. BB-500 had the lower NDF concentration compared to the other inoculant treatments within the 24 and 48 post-harvest ensiling time, respectively. There was a rate effect in NDF concentration (P = 0.0090) in which the 1X had a slightly greater concentration (Fig. 2). A significant difference in NDF concentration was observed among three ensiling periods (P <0.0001), with increase in NDF from 30 to 60 d ensiling (7.5%) (Fig. 3). A significant inoculant treatment x ensiling period interaction was observed in NDF concentration (P < 0.0001). Fiber (NDF) concentrations increased with ensiling period within each inoculant treatment, but significant differences among inoculant treatments was observed for the 30 and 40 d ensiling period (Table 4). Fiber concentration increased within each inoculant treatment across the three ensiling periods. A post-harvest ensiling time x ensiling period interaction (P = 0.0116) indicated that 24 h post-harvest ensiling had lower NDF concentration at the 45 d, but higher at 60 d ensiling period. No differences were observed among post-harvest ensiling times at the 30 d ensiling period (Table 5).
| Ensiling Period (d) | ||||
| Post-harvest Ensiling (h) | 30 | 45 | 60 | LSD0.05 |
| ---------------------------------- pH ---------------------------------- | ||||
| 24 | 4.09 | 4.67 | 4.47 | 0.14 |
| 48 | 4.13 | 4.89 | 4.85 | 0.18 |
| LSD0.05 | NS | 0.18 | 0.19 | |
| ---------------------------------- ºBrix (%) ---------------------------------- | ||||
| 24 | 14.34 | 14.15 | 12.85 | 0.49 |
| 48 | 23.99 | 22.91 | 20.51 | 1.01 |
| LSD0.05 | 0.72 | 0.80 | 0.86 | |
| ---------------------------------- CP (%)---------------------------------- | ||||
| 24 | 14.08 | 13.07 | 13.70 | 0.26 |
| 48 | 12.95 | 12.86 | 13.90 | 0.21 |
| LSD0.05 | 0.25 | NS | NS | |
| ---------------------------------- ADF (%) ---------------------------------- | ||||
| 24 | 29.26 | 30.93 | 32.47 | 0.38 |
| 48 | 30.24 | 30.83 | 32.09 | 0.27 |
| LSD0.05 | 0.34 | NS | 0.34 | |
| ---------------------------------- NDF (%) ---------------------------------- | ||||
| 24 | 44.57 | 45.90 | 48.28 | 0.64 |
| 48 | 44.38 | 44.84 | 47.46 | 0.60 |
| LSD0.05 | NS | 0.73 | 0.51 | |
| ---------------------------------- IVTDMD (%) ---------------------------------- | ||||
| 24 | 84.50 | 82.74 | 83.11 | 0.46 |
| 48 | 83.74 | 83.50 | 84.40 | 0.30 |
| LSD0.05 | 0.38 | 0.30 | 0.48 | |
In vitro True Dry Matter Digestibility (IVTDMD) – Dry matter digestibility (DMD) is usually the portion of the annual ryegrass baleage that could be digested by livestock at specific level of forage intake. Due to IVTDMD being a very expensive and laborious analysis, DMD is frequently estimated by NIR. Differences were observed among microbial inoculants (P < 0.0001), in which BH had higher digestibility (Table 2). Differences in post-harvest ensiling times (P <0.0001) were observed with the 48 h post-harvest ensiling having a slightly higher digestibility (Fig. 1). A significant inoculant treatment x post-harvest ensiling time interaction (P = 0.0002) indicated that most of the differences in DMD were attributed to differences among inoculants within each post-harvest ensiling time (Table 3). Differences among post-harvest ensiling times were observed only for BB-40788 and BH. Significant differences in DMD was observed among the inoculant application rate (P = 0.0125), but those differences were very small (Fig. 2). Differences among ensiling periods (P <0.0001) also indicated that the 30 d had higher DMD compared to the other two ensiling periods (Fig. 3). A post-harvest ensiling time x ensiling period interaction (P <0.0001) indicated that 24 h post-harvest ensiling had higher DMD concentration at the 30 d, but lower at the 45 and 60 d ensiling period, respectively (Table 5).
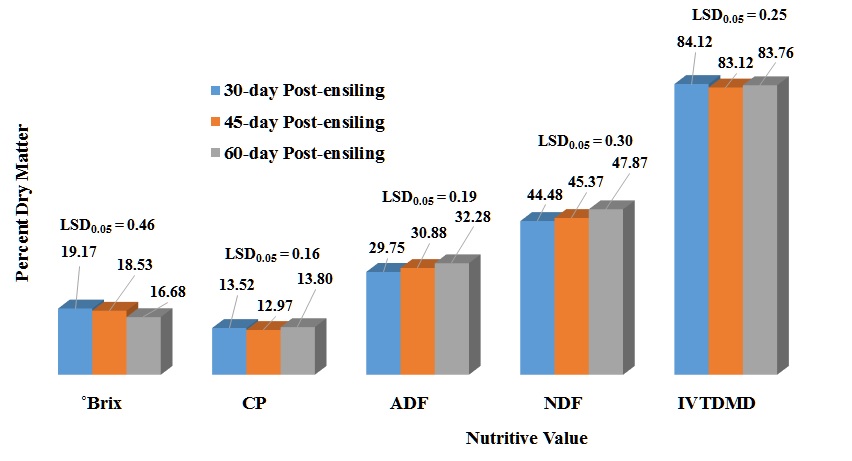
Figure 3. Influence of ensiling period on ºBrix and nutrirtive value of annual ryegrass baleage.
Conclusions
The need to preserve forages with higher nutritive value has increased due to rising commodity prices. Allowing forage fermentation to occur with an inoculant that promotes a rapid pH decline could represent a significant risk for dry matter loss as well as production of undesirable end products such as butyric acid. Forage inoculants can help ensure baleage reaches the correct pH range and acid profile to promote stability, retain DM and maximize nutrient preservation. Some of the microbial inoculants used in this study improved the fermentation of annual ryegrass baleage, as evidenced by the lower pH after 30 d of ensiling. Nevertheless, the pH of Biotal Buchneri 500 and Biotal Buchneri 40788 decreased more rapidly than those of other microbial inoculants tested in this study.
This study indicates that microbial inoculants containing only homolactic bacteria or those containing homolactic and heterolactic bacteria can be used to improve the fermentation and aerobic stability of annual ryegrass baleage. In some cases, pH was lower than that observed for untreated forage at 30 d of ensiling when microbial inoculants were used. This in turn suggests that particular care in selecting and using inoculants will be required when deciding to inoculate annual ryegrass baleage. Microbial inoculants that did not contain Lactobacillus buchneri did not lower the pH and improve the quality of the baleage. With such a good natural fermentation of annual ryegrass, it is difficult for an inoculant to make substantial improvements in fermentation. These results relate to the inoculants and the forage examined in this study and more studies are needed in large scale round bales under typical climatic conditions during the peak of annual ryegrass production in the southern USA.
Inoculants are not effective in all harvesting situations, as they do not always enhance fermentation. The use of a microbial inoculant should always be associated with good management practices. Following these management practices will improve ensiling and increase the effectiveness of an inoculant. The effectiveness of the use of a baleage inoculant is dependent on the market situation, prices, and environmental conditions in the farm. Field curing temperature, moisture content, and wilting time of the silage have an effect on inoculant success. It is important to remember that these factors change each day. All factors must be considered before making the decision to use inoculants in a given situation. A knowledge of how and when inoculants work should be considered and used as an orientation in order to help producers in the decision to use baleage inoculants, or in order to estimate the potential profit.
Literature Cited
Adesogan, A.T., Salawu, M.B., Ross, A.B., Davies, D.R., and Brooks, A.E. (2003). Effect of Lactobacillus buchneri, L. fermentum or Leuconostoc mesenteroides inoculants or a chemical additive on the fermentation, aerobic stability and nutritive value of crimped wheat grains. Journal of Dairy Science, 86: 1789-1796.
Adesogan, A.T. (2006). Factors affecting corn silage quality in hot, humid climates. Proc.17th Annual Florida Ruminant Nutrition Symposium, Gainesville, Florida, January, 2006. pp. 108-119.
Adesogan, A.T. (2008). Recent Advances in Bacterial Silage Inoculant Technology. In: Proceedings of the Florida Ruminant Nutrition Symposium, January 30. Gainesville, FL. p. 1-17.
Allen, V. G., Batello, C., Berretta, E. J., Hodgson, J., Kothmann, M, Li, X., McIvor, J., Milne, J., Morris, C., Peeters, A., and Sanderson, M. (2011). An international terminology for grazing lands and grazing animals. Grass and Forage Science, 66: 22-28.
Arriola, K.G., Kim, S.C. and Adesogan, A.T. (2011). Effect of applying inoculants with heterolactic or homolactic and heterolactic bacteria on the fermentation and quality of corn silage. Journal of Dairy Science, 94: 1511-1516.
Buckmaster, D. and Lundmark, D. (1992). Bacterial Inoculants for Silage. Pennsylvania Cooperative Extension Service, The Pennsylvania State University, Publication I-111.
Collins, M., and Owens, V.N. (2003). Preservation of forage as hay and silage. In Forages: an introduction to grassland agriculture. In: R.F. Barnes, C.J. Nelson, K.J. Moore and M. Collins, eds. Blackwell Publishing, Ames, IA. pp. 443-471.
Driehuis, F., Oude Elferink, S.J.W.H. and Van Wikselaar, P.G. (2001). Fermentation characteristics and aerobic stability of grass silage inoculated with Lactobacillus buchneri, with or without homofermentative lactic acid bacteria. Grass Forage Science, 56: 330-343.
Filya, I., Sucu, E., and Karabulut, A. (2004). The effect of Propionibacterium acidipropionici, with or without Lactobacillus plantarum, on the fermentation and aerobic stability of wheat, sorghum and maize silages. Journal of Applied Microbiology, 97: 818-826.
Kleinschmit, D.H. and Kung, L. Jr. (2006). A meta-analysis of the effects of Lactobacillus buchneri on the fermentation and aerobic stability of corn and grass and small-grain Silages. Journal of Dairy Science, 89: 4005-4013.
Krooneman, J., Faber, F., Alderkamp, A.C., Oude Elferink, S.J.H.W., Driehuis, F., Cleenwerck, I., Swings, J., Gottschal, J.C., and Vancanneyt, M. (2002). Lactobacillus diolivorans sp. Nov., a 1,2-propanediol-degrading bacterium isolated from aerobically stable maize silage. International Journal of Systematic and Evolutionary Microbiology, 52: 639-646.
Kung, L. Jr., Taylor, C.C., Lynch, M.P., and Neylon, J.M. (2003a). The effect of treating alfalfa with Lactobacillus buchneri 40788 on silage fermentation, aerobic stability, and nutritive value for lactating dairy cows. Journal of Dairy Science, 86: 336-343.
Kung, L. Jr., Stokes, M.R., and Lin, C.J. (2003b). Silage additives. In: D.R. Buxton, R.E. Muck, and J.H. Harrison, eds. Silage science and technology. ASA-CSSA-SSSA Publishers, Madison, WI. pp. 305-360.
McCormick, M.E., Cuomo, G.J., and Blouin, D.C. (1998). Annual ryegrass stored as balage, haylage, or hay for lactating dairy cows. Journal of Production Agriculture, 11 (3): 293-300.
McCormick, M. (2013). Baled Silage – Uses for Beef and Dairy. In: Proceedings of the Southern Pasture and Forage Crop Improvement Conference. April 22-24. Tyler, TX. pp. 12-15.
Moshtaghi, S.A. and Wittenberg, K.M. (1999). Use of Forage Inoculants with or without Enzymes to Improve Preservation and Quality of Whole Crop Barley Forage Ensiled as Large Bales. Canadian Journal of Animal Science, 79 (4): 525-532, https://doi.org/10.4141/A99-019.
Ranjit, N.K. and Kung, L. Jr. (2000). The effect of Lactobacillus buchneri, Lactobacillus plantarum, or a chemical preservation on the fermentation and aerobic stability of corn silage. Journal of Dairy Science, 83: 526-535.
Ranjit, N.K., Taylor, C.C., and Kung, L. Jr. (2002). Effect of Lactobacillus buchneri 40788 on the fermentation, aerobic stability and nutritive value of maize silage. Grass Forage Science, 57: 73-81.
Romero, J.J., Zhao, Y., Balseca-Paredes, M.A., Tiezzi, F., Gutierrez-Rodriguez, E., and Castillo, M.S. (2017). Laboratory silo type and inoculation effects on nutritional composition, fermentation, and bacterial and fungal communities of oat silage. Journal of Dairy Science 100: 1812–1828. https://doi.org/10.3168/jds.2016-11642
Weinberg, Z.G., and Muck, R.E. (1996). New trends and opportunities in the development and use of inoculants for silage. FEMS Microbiology Reviews, 19: 53-68.
Weinberg, Z.G., Szakacs, G., Ashbell, G. and Hen, Y. (1999). The effect of Lactobacillus buchneri and L. plantarum, applied at ensiling, on the ensiling fermentation and aerobic stability of wheat and sorghum silages. Journal of Industrial Microbiology and Biotechnology, 23: 218-222.
Acknowledgement
This publication is a contribution of Mississippi Agricultural and Forestry Experiment Station and Mississippi State University Extension Service. Thank you to student workers (Isaac Picket, Daniel Moore, Derek McCain, William M. Hammack, and Kyle Munn) for assisting with data collection and sample processing and analysis. Thank you to Biotal (Lallemand Animal Nutrition, Milwaukee, WI), ProbioFerm (ProbioFerm, Ltd., Des Moines, IA) and Ecosyl (Ecosyl Products Inc., Byron, IL) for donating the products for this study.
Disclaimer
Mention of a trademark, proprietary product, or vendor does not constitute a guarantee or warranty of the product by Mississippi State University and does not imply its approval to the exclusion of other products or vendors that also may be suitable.
