Journal of the NACAA
ISSN 2158-9429
Volume 4, Issue 1 - June, 2011
Effect of Soil Amendments on Soil Enzyme Activities and Active Carbon on a Managed Douglas-Fir Forest Ecosystem
- Angima, S.A., Assistant Professor, Oregon State University Extension
Bouldin, J.L., Assistant Professor, Arkansas State University
Green, V.G., Associate Professor, Arkansas State University
Woodruff, T., Graduate Student, Arkansas State University
ABSTRACT
Effects of soil amendments on soil enzyme activities and active carbon were examined on a 25-year old forested ecosystem consisting of Douglas-fir (Pseudotsuga menziesii [Mirb.] Franco) in Central Western Oregon. Five treatments were developed based on most common forest practices. The treatments were: full soil test recommended rates with weed control, half soil test recommended rates with weed control, full soil test recommended rates without weed control, weed control only, and the control. These recommendations were applied in 2004, 2005, and 2006. Soil samples were collected in 2007 and analyzed for pH, organic matter, three soil enzyme activities, and active carbon (C). Enzyme analyses were β-glucosidase, arylsulfatase, and acid phosphatase. pH range was 5.1 to 5.5 and organic matter was between 5% and 6%. Although control plots resulted in greater numerical values, no statistically significant differences among the treatments for the measured enzyme activities or active C were noted, except for β-glucosidase between the control and the full recommended rate treatments. Adding soil amendments to forest ecosystems helps boost nutrient content of soils and does not adversely suppress the microbial community responsible for nutrient cycling in the short term.
Keywords: Soil quality, weed control, β-glucosidase, arylsulfatase, acid phosphatase.
Introduction
A healthy soil will have a high and diverse population of beneficial organisms, enabling the mineralization of soil organic matter into inorganic forms of plant nutrients available for plant uptake (Gugino et al. 2007). Dominant among these soil organisms are bacteria, fungi, protozoa, nematodes, and the larger invertebrate species millipedes, snails, and slugs (Smith and Smith 2006). Soil enzymes are extracellularly produced by these microorganism communities, significantly contributing to nutrient cycling and soil quality.
Soil quality can broadly be defined as the ability of soil to support a healthy microbial community capable of recycling nutrients necessary for plant growth and sustain the demands of plant and animal needs (Gugino et al. 2007). Nutrient cycling involves the flow from the nonliving to the living and back to the nonliving components of an ecosystem. Some indicators of soil quality include soil enzymes and active carbon (active C). Soil enzymes produced by microorganisms and plant root systems are the catalysts through which the reactions of the nutrient cycles occur (Speir and Ross 1978, Green et al. 2007). Changes in enzyme activity can be observed within thirty days to three years after application of soil amendments (Dick 1994). Active C, which is a small and labile component of soil organic carbon, can be used as an indicator of changes in soil quality in response to soil management practices (Weil et al. 2003). All steps in a nutrient cycle are interdependent and the time it takes for a nutrient to cycle through any given system varies greatly depending on the amount of nutrient available, water, climate, and organisms (Smith and Smith 2006).
Nutrient management of soils has been practiced in agriculture for many years, but is less common in forestry (Gugino et al. 2007). Illmer and Schinner (1991), studied forest soils in pots in an incubation study and following lime and mineral salts application. Microbiological activity was initially suppressed but over time, increased in the treated pots compared to control treatments. Another forest soil study byAcosta-Martinez and Harmel (2006)included the application of poultry litter to forest soils. They measured increased enzyme activities following poultry litter application.
Commonly measured soil enzyme activities include β-glucosidase, arylsulfatase, and acid phosphatase enzymes. β-glucosidase plays a major role in the breakdown of carbohydrates in soils. β-glucosidase catalyzes the hydrolysis of carbohydrates where H is cleaved from water molecules and smaller carbohydrate chains are formed, eventually resulting in glucose as a metabolically usable form of C. Additionally, the sequence of reactions in this cycle is exergonic, producing energy for soil microorganisms (Eivazi and Tabatabai 1988; Nelson and Cox 2000). Arylsulfatase is one of the many sulfatase enzymes that act to releases SO42-, the bioavailable form of sulfur (S) for plant uptake in the S cycle. Arylsulfatase catalyzes the hydrolysis of ester-sulfate, which is a major source of S in many soils (Dick et al. 1996). Acid phosphatase helps complete the phosphorous (P) cycle by catalyzing ester-phosphate hydrolysis producing PO43- as an end product (Dick 1994). The form of P available to the plant for uptake is based on the pH of the soil, however, the released PO43- will react within the soil to produce the plant-available forms, H2PO4- or HPO42- (Havlin et al. 2005).
Active C was studied for its importance in the soil food web, which greatly affects nutrient cycles. Active C is a small portion of SOC, but is labile and may be able to provide an early indicator of changes in soil quality in response to soil management practices due to its ability to cycle more quickly (Weil et al. 2003).
The objectives of this study were to determine the effect of soil amendments, as a tool in forest management, on soil enzyme activities and active C. Results of this study will allow forest managers to gauge whether inorganic amendments of soil promote or hinder the development of active soil microorganism communities that in turn may affect how nutrients are cycled in a forestry ecosystem.
Materials and Methods
Site Description
The study site was Starker Forest Inc. which is located in western Oregon, USA, approximately 32 km east of Newport, Oregon (Starker Forests 2008), near the Pacific Ocean (44° 40' N and 123° 38') at 200 M above sea level. The climate is cool and wet during fall, winter, and spring while the summer months are dry and warm. Average temperatures range from approximately 4 °C in the winter months to approximately 27 °C in the summer months (NOAA 2008). Precipitation ranges from approximately 17.8 cm per month in the winter months to less than 2.5 cm per month in the summer months June to September (NOAA 2008). The forest is 100% Douglas-fir and the soil is predominately Apt-McDuff silty clay loams (fine, isotic, mesic Typic Haplohumults) on a 5-30% slope (USDA 2008). The study site was divided into 10 plots that were 0.1 hectare each (Figure 1). In establishing the plots, areas within the study site we chosen that had gentle gradient (5%) and more representative of the productive sections of the forest with the Apt-McDuff silt clay loams. Treatments were then assigned randomly to these ten plots.
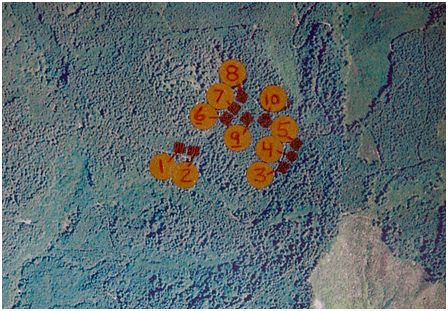
Figure 1 Plot locations inside Starker Forest, Oregon, USA (44° 40' N and 123° 38'). Lime and Fertilizer treatments included: Plot 1 & 8 - ½ recommended rates with weed control; Plots 2 & 10 - full recommended rate with weed control; Plots 3 & 7 - full recommended rate with no weed control; Plots 4 & 9 - control; and Plots 5 & 6 - weed control only.
Soil Sampling
Initial soil sampling was done at a depth of 0-30 cm in 2004 using a soil probe, composited, and sent for analysis. The resulting soil test recommendations were used to apply lime and fertilizer amendments for the five treatments which were; full recommended rate with weed control, half recommended rate with weed control, full recommended rate with no weed control, control, and weed control (glyphosate, isopropylamine salt) only. The amendments were applied in 2004, 2005 and 2006, during the period April to June (Table 1). The treatments were replicated twice. Recommended nutrient applications (Table 1) included sulfur (S), phosphorous (P), lime (CaCO3, CaMg(CO3)2), potassium (K), boron (B), iron (Fe), copper (Cu), and zinc (Zn).
Table 1. Lime and fertilizer rates applied to a Douglas-fir forest ecosystem consisting of five treatments in Western Oregon, USA, during 2004-2006.
|
Plot |
Treatment |
S |
P |
Lime |
K |
B |
Fe |
Cu |
Zn |
|
|
|
----------(Kg ha-1)----------- |
----------(ppm)--------- |
||||||
|
1 |
½ recommended rate w/weed control |
50 |
140 |
4,172 |
47 |
1.2 |
47 |
3.86 |
5 |
|
2 |
Full recommended rate w/weed control |
100 |
280 |
8,345 |
94 |
2.4 |
94 |
7.7 |
10 |
|
3 |
Full recommended rate no weed control |
100 |
280 |
8,345 |
94 |
2.4 |
94 |
7.7 |
10 |
|
4 |
Control |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
5 |
Weed control only |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
6 |
Weed control only |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
7 |
Full recommended rate no weed control |
100 |
280 |
8,345 |
94 |
2.4 |
94 |
7.7 |
10 |
|
8 |
½ recommended rate w/weed control |
50 |
140 |
4,172 |
47 |
1.2 |
47 |
3.86 |
5 |
|
9 |
Control |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
10 |
Full recommended rate w/weed control |
100 |
280 |
8,345 |
94 |
2.4 |
94 |
7.7 |
10 |
In May 2007, soil samples (0-10 cm) were collected randomly from each plot, composited and placed in separate resealable plastic bags and shipped in coolers with ice packs to the Arkansas State University Soils Laboratory where they were stored at 4°C. These samples were later placed in paper bags to air dry, then crushed and gently sieved through a 2-mm sieve. The resultant samples were then placed in polyethylene cups and stored at room temperature until analyzed.
Soil Analyses
β-glucosidase, arylsulfatase, acid phosphatase, and active carbon were analyzed for each sample. β-glucosidase was analyzed using the method of Eivazi and Tabatabai (1988). The supernatant from the incubated sample was evaluated on a Shimadzu UVmini-1240 UV-spectrophotometer (Shimadzu, Columbia, MD) at 410 nm. Absorbance was compared to a calibration curve.
The acid phosphatase analysis followed the Eivazi and Tabatabai (1977)procedure and arylsulfatase activity was analyzed using the procedure of Tabatabai and Bremner (1970). The filtered supernatant was evaluated in the same manner as β-glucosidase with absorbance being compared to a calibration curve.
Active carbon analyses were performed following the protocol described in Weil et al. (2003) with minor adaptations from correspondence with the author (Ray Weil, pers. Comm., University of Maryland – College Park, November 16, 2007). Air-dried soil (2.5 g) was weighed into 50-mL polypropylene centrifuge tubes. Twenty mL of KMnO4 was then added to each tube and the contents were hand shaken for 120 seconds. The tubes were then inverted and the soil was allowed to settle for 10 minutes. During this time, a second set of 50-mL centrifuge tubes was filled with 45 mL of deionized water. A 0.50-mL aliquot of solution was extracted from the top 1 cm of the first tube and placed into the second tube. The second tube was then filled to the 50-mL mark with deionized water and inverted several times to mix. The absorbance of the resulting solution was measured on a Shimadzu UVmini-1240 spectrophotometer (Shimadzu, Columbia, MD) at 550 nm.
β -glucosidase, arylsulfatase, and acid phosphatase analyses were run in duplicate. A control was run on each sample set, in order to allow for yellow coloring that was not derived from enzyme activity. For sample controls, the procedure for each enzyme remained the same, but the addition of 1 ml of substrate in each method was made after the incubation period. Active C was run in duplicate.
Statistical Methods
Quality control for test acceptability of enzyme analyses included calculation of the coefficient of variation between the analytical replicates. Test runs were deemed acceptable if this variance was less than 10% for the replicates. Active carbon was analyzed with two replicates and the calculated coefficient of variation between the two replicates for active C was deemed acceptable if the coefficient of variation was less than 5%, a standard suggested by the author (Weil 2008). A one-way ANOVA (Minitab™ 2000)was used to determine variance among the treatments for all analyses. A Dunnett’s ANOVA (Minitab™ 2000)was used to compare β-glucosidase treatments to the control. Because β-glucosidase and active C are both part of the C cycle, a correlation analysis was used to determine if there was a significant correlation.
Results and Discussion
β-glucosidase
β-glucosidase enzyme activity ranged from 73.2 to 101 µg p-nitrophenol g-1 soil h-1 (Figure 2). Overall, the control treatment had greater measured activity. However, there was no statistical difference among treatment types at α=0.05 (p=0.089) except between the control and the full recommended rate with weed control treatment using Dunnett’s ANOVA. The lack of significant differences in β-glucosidase enzyme activity between full recommended rate without weed control and the control suggests that the understory vegetation may have an impact on microbial activity in forest ecosystems.
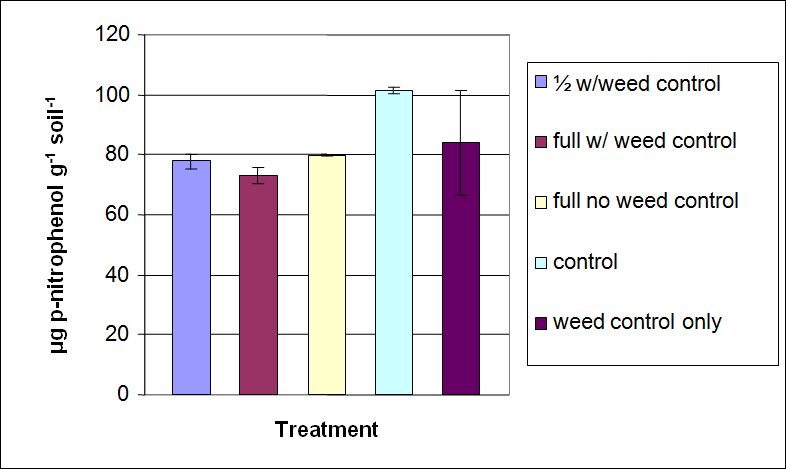
Figure 2. β-glucosidase activity measured in soils sampled from treatment plots at Starker Forest Inc., Oregon, USA. Bars represent mean value ± SD.
Eivazi and Tabatabai (1990) studied the β-glucosidase enzyme to determine the effects of its activity by investigating a number of inorganic trace elements common in soil management practices. They determined that many inorganic additives inhibit the soil enzyme in some measure. The inorganic trace elements B, Zn, Fe, and Cu were all shown in Eivazi and Tabatabai’s study to be inhibitors of β-glucosidase, and these inorganic elements were applied to the soil at Starker Forest Inc. These inorganic elements are described as non-competitive inhibitors, attaching to the enzyme at a place other than the active site and altering the shape of the enzyme (Eivazi and Tabatabai 1990; Nelson and Cox 2000). Non-competitive inhibition will decrease the rate of catalyzation of an enzyme, therefore slowing the hydrolysis of carbohydrates. In this study, while there were no statistical differences observed among the treatments, greater enzyme activity in the control treatment, which had no inorganic nutrients applied, was noticeably greater for β-glucosidase enzyme activity than the other treatments. Our observations are consistent with the findings of Eivazi and Tabatabai (1990).
Arylsulfatase
Arylsulfatase enzyme activity ranged from 455 to 646 µg p-nitrophenol g-1 soil h-1 (Figure 3). The greatest activities were observed in the control and weed control only treatments. However, there were no statistically significant differences among treatments at α=0.05 (p= 0.373). Arylsulfatase was shown in this study to have a numerically decreased enzyme activity in micronutrient treated soil as compared to the control and weed control only treatments. Other research has demonstrated the effects of trace elements on the activity of the arylsulfatase enzyme (Al-Khafaji and Tabatabai 1979). The research showed that although most inorganic trace elements added to the soil provided some inhibition, B was a very effective inhibitor followed by Cu and Fe. The inorganic elements are non-competitive inhibitors, binding to the –SH group of an enzyme and altering the shape of the enzyme (Al-Khafaji and Tabatabai 1979), slowing catalysis, but do not stop the enzyme from catalyzing reactions (Nelson and Cox 2000). Since arylsulfatase is a major factor in the mineralization of S, the entire S cycle would be slowed (Tabatabai and Bremner 1970). These reported results are consistent with the results of this study as B, Cu, and Fe were included as inorganic trace elements in the treatments.
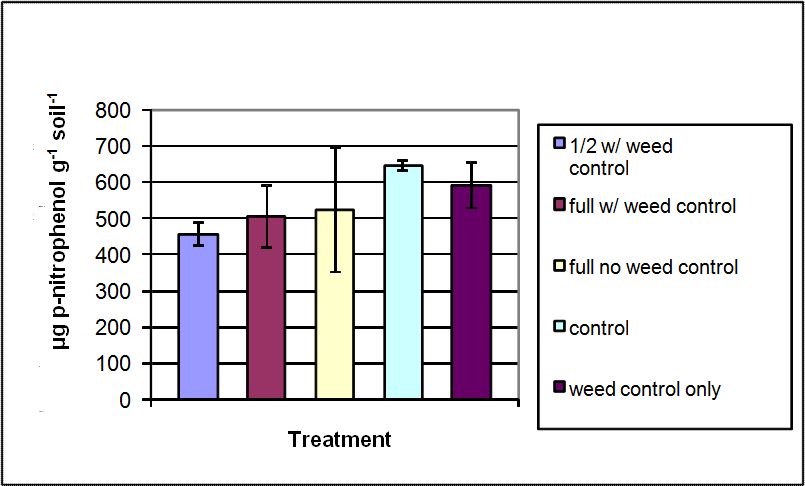
Figure 3. Arylsulfatase activity measured in soils sampled from treatment plots of the Starker Forest Inc., Oregon, USA. Bars represent mean value ± SD.
Acid phosphatase
Acid phosphatase enzyme activity ranged from 505 to 773 µg p-nitrophenol g-1 soil h-1 (Figure 4). The greatest activities were seen in the ½ recommended rate with weed control and the control treatments, while the least activity was seen in the full recommended rate with weed control treatment. No statistically significant differences among treatments were observed at α=0.05 (p= 0.416). Speir and Ross (1978) measured the inhibition of acid phosphatase following the addition of inorganic P, Cu, and Zn. In this study, the greater acid phosphatase activity was observed in soils from the control plots, thus supporting the Speir and Ross study. While there was no statistically significant difference, the ½ recommended rate with weed control had numerically similar results as the control.
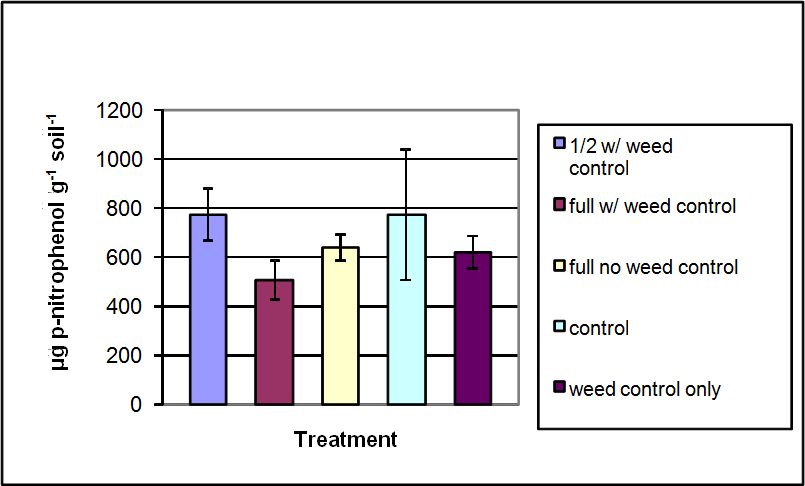
Figure 4. Acid phosphatase activity measured in soils sampled from treatment plots of the Starker Forest Inc., Oregon, USA. Bars represent mean value ± SD.
Active Carbon
Active C concentrations in the soils ranged from 639 to 786 mg kg-1 (Figure 5). The control treatment exhibited a numerically greater mean than the other treatments. However, there were no statistically significant differences among treatments at α=0.05 (p= 0.144). The concentration of active C was observed to be less in the treatments than in the control, similar to β-glucosidase.
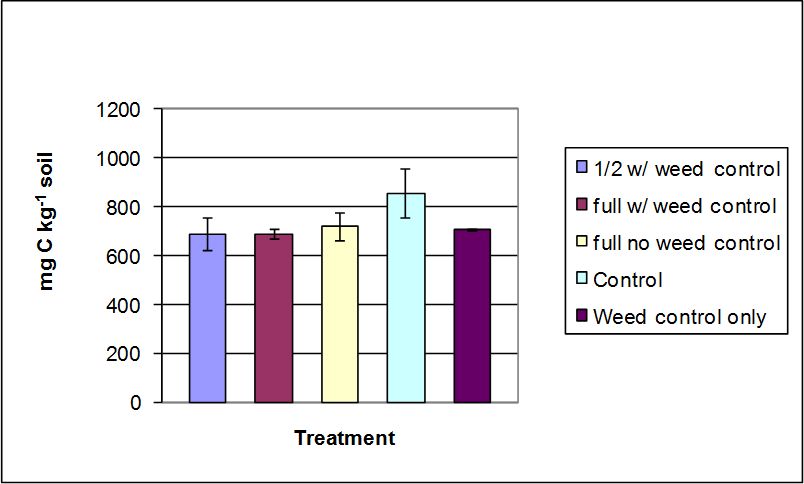
Figure 5. Active carbon concentrations measured in soils sampled from treatment plots of the Starker Forest, Oregon, USA. Bars represent mean value ± SD.
This pattern has been observed in earlier studies in which the activity of β-glucosidase was strongly correlated with organic C (Eivazi and Tabatabai 1990). A correlation analysis (Figure 6) of β-glucosidase and active C also indicated a statistically significant correlation (α=0.05) between β-glucosidase and active C (r=0.692, p=0.027). Because β-glucosidase is important in the breakdown of energy-rich carbohydrates, it can be assumed that the entire C cycle would be affected.
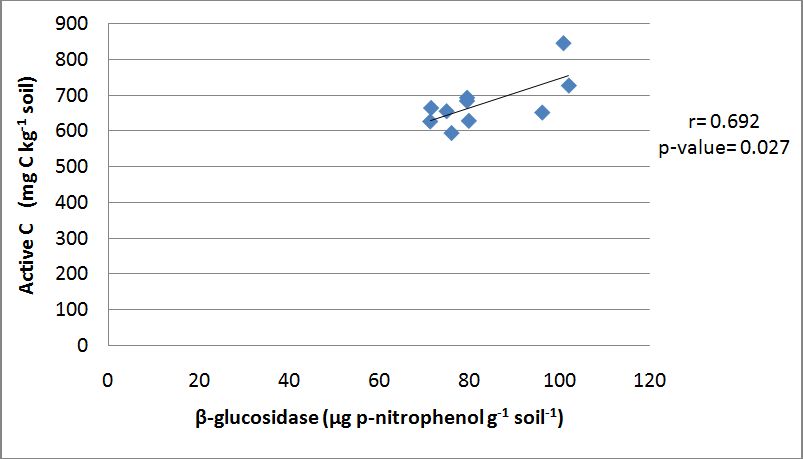
Figure 6. Correlation measured between active C concentration and β-glucosidase activity at Starker Forest Inc, Oregon, USA.
The addition of inorganic elements may also affect the activity of enzymes, both positively and negatively, by changing the pH of soils. Enzyme activity is maximized at a neutral soil pH (Nelson and Cox 2000)but the pH of a soil can be varied drastically by the addition of different inorganic elements (Al-Khafaji and Tabatabai 1979). Lime, which is used to make a soil more basic, was used in two different forms on the soil at Starker Forest in both 2004 and 2005. Lime forms were calcium carbonate and calcium magnesium carbonate. pH and organic matter content (in 2007) for all treatments are shown on Figure 7. In this study, the pH of the soil in all treatments varied between 5.1-5.5 (Figure 7). This small range in pH may not significantly contribute to observed differences in enzyme activity between the control and other treatments.
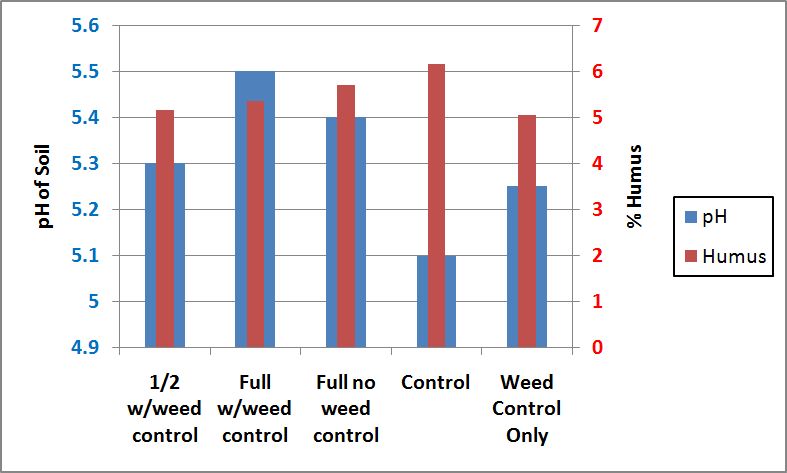
Figure 7. pH and humus content (in 2007) for all treatments at Starker Forest Inc, Oregon, USA.
Overall, enzyme activity and active C resulted in a lower level of activity in each treatment compared to the control treatment, although measured activities were not significantly different. Other studies have also indicated that the addition of inorganic elements to the soil can result in inhibition of enzyme activities. Lime indirectly affects enzyme activities by drastically altering the pH of the soil and therefore changing soil reactions (Al-Khafaji and Tabatabai 1979; Nelson and Cox 2000; Havlin et al. 2005). In a study by Perucci (1992), municipal sewage waste was used as nutrient treatments to soils. Activities of enzymes were shown to be increased within 30 days and greater activities continued to be observed for three years (Perucci 1992). While these are organic treatments, it has been noted that once a particular management practice has been established, enzyme activities tend to stabilize over time (Dick 1994). In the forest tillage study by Dick in western Oregon, a treatment effect was observed in β-glucosidase activity even after two years following disturbance (Dick 1994). Since this was a tillage study, the effects may be observed more quickly due to the even distribution of the soil during tillage. In addition to treatment applications, the temperate nature of Oregon’s climate could be a contributing factor in the amount of time necessary to observe potential differences in enzyme activity due to management.
Conclusion
Up to three years following application of lime and fertilizer amendments in a forest ecosystem, no statistically significant differences in soil quality indicators were observed among the control and amended soils except for β-glucosidase enzyme activity where full fertilizer recommendations with weed control were applied compared to the control treatment. β-glucosidase enzyme activity is associated with carbohydrates in soils and is part of the carbon cycle. However, no significant differences were observed in active carbon levels, an early indicator of changes in soil quality. Since there was only a slight decrease in soil microbial activity as indicated by other soil enzyme analyses that control the S and P cycles, it will be appropriate to conclude that adding soil amendments to forest ecosystems can boost soil nutrient status and at the same time support considerable soil microorganism communities useful in nutrient cycling. Literature review reveals that in the long-term, enzyme activity stabilizes with time with fertilizer applications for both crop and forest ecosystems. This study provides a basis for encouraging fertilizer and lime application to forest ecosystem if soil tests indicate a need but also shows that microbial enzyme activities responsible for nutrient cycling are not critically affected when fertilizer and lime are added.
Acknowledgements
The authors appreciate the partnership between Oregon State University Extension, Arkansas State University and Starker Forests Inc. that enabled the completion of this study.
Literature Cited
Minitab Inc. 2000. Release 13, State College, PA.
Acosta-Martinez, V., and R.D. Harmel. 2006. Soil microbial communities and enzyme activities under various poultry litter application rates. Journal of environmental Quality 35:1309-1318.
Al-Khafaji, A.A., and M.A. Tabatabai. 1979. Effects of trace elements on arylsulfatase activity in soils. Soil Science 127:129-133.
Dick, R.P. 1994. Soil enzyme activities as indicators of soil quality. P. 107-124 in Defining Soil Quality for A Sustainable Environment; SSSA Special Publication Number 35, Doran, J.W., D.C. Coleman, D.F. Bezdiceck, and B.A. Stewart (eds.). Soil Science Society of America, Madison, WI.
Dick, R.P., D.P. Breakwell, and R.F. Turco. 1996. Soil enzyme activities and biodiversity measurements as integrative microbiological indicators, P. 247-271, in Methods for Assessing Soil Quality Vol. 49, Doran, J.W., and A. J. Jones, (eds.). Soil Science Society of America, Madison, WI.
Eivazi, F., and M.A. Tabatabai. 1977. Phosphatases in soils. Soil Biology and Biochemistry 9:167-172.
Eivazi, F., and M.A. Tabatabai. 1988. Glucosidases and galactosidases in soils. Soil Biology and Biochemistry 20:601-606.
Eivazi, F., and M.A. Tabatabai. 1990. Factors affecting glucosidase and galactosidase activities in soils. Soil Biology and Biochemistry 22:891-897.
Green, V.S., D.E. Stott, J.C. Cruz, and N. Curi. 2007. Tillage impacts on soil biological activity and aggregation in a Brazilian Cerrado Oxisol. Soil and Tillage Research 92:114-121.
Gugino, B.K., O.J. Idowu, R.R. Schindelbeck, H.M. van Es, D.W. Wolfe, B.N. Moebius, J.E. Thies, and G.S. Abawi. 2007. Cornell Soil Health Assessment Training Manual.
Havlin, J.L., J.D. Beaton, S.L. Tisdale, and W.L. Nelson. 2005. Soil fertility and fertilizers: An introduction to nutrient management. Prentice Hall, Upper Saddle River, NJ. ISBN 0-13-626806-4.
Illmer, P., and F. Schinner. 1991. Effects of lime and nutrient salts on the microbiological activities of forest soils. Biology and Fertility of Soils 11:261-266.
Nelson, D.L., and M.M. Cox. 2000. Lehninger Principles of Biochemistry. Worth Publishers, New York, NY.
NOAA. 2008. Available online at www.noaa.gov; last accessedDecember 08, 2008).
Perucci, P. 1992. Enzyme activity and microbial biomass in a field soil ammended with municipal refuse. Biology and Fertility of Soils 14:54-60.
Smith, R.L., and T.M. Smith. 2006. Elements of ecology. Benjamin Cummings, San Francisco, CA.
Speir, T.W., and D.J. Ross. 1978. Soil Phosphatase and Sulphatase, P. 380, in Soil Enzymes, Burns, R.G. (ed.). Academic Press, New York, New York.
Starker Forests. 2008. Available online at www.starkerforests.com; last accessed December 08, 2009).
Tabatabai, M.A., and J.M. Bremner. 1970. Arylsulfatase activity of soils. Soil Science Society of America Proceedings 34:225-229.
USDA. 2008. Web Soil Survey. Available online at http://websoilsurvey.nrcs.usda.gov/app/; last accessed December 08, 2009).
Weil, R.R. 2007. Personal Communication. University of Maryland – College Park, November 16, 2007.
Weil, R.R., K.R. Islam, M.A. Stine, J.B. Gruver, and S.E. Samson-Liebig. 2003. Estimating active carbon for soil quality assessment: A simplified method for laboratory and field use. American Journal of Alternative Agriculture 18:3-17.
