Journal of the NACAA
ISSN 2158-9429
Volume 11, Issue 1 - June, 2018
Year-round Control of Russian Olive Using the Cut-Stump Treatment
- Patterson, R. K., Agriculture/4-H Youth Agent, Utah State University
Worwood, D. R., Agriculture/4-H Youth Agent, Utah State University
Price, S., Agriculture/4-H Youth Agent, Utah State University
ABSTRACT
Russian olive (Elaeagnus angustifolia) is an invasive tree impacting agricultural areas and disrupting riparian system functions. An herbicide treatment to stumps remaining after tree removal, known as a “cut-stump treatment”, may be a viable long-term alternative to problematic mechanical treatments, which stimulates growth from epicormic and adventitious buds. Monthly treatments were made across a three-year period in Carbon County Utah to assess control efficacy throughout the year. No regrowth was observed on herbicide treated stumps during the treatment year and only 4% of stumps exhibited regrowth two years later compared to the 97% of controls which developed regrowth.
Introduction
Russian olive (Elaeagnus angustifolia), once touted as a great windbreak, erosion control and wildlife habitat plant, is now known for its overwhelming colonization abilities and negative environmental impacts throughout the western United States and Canada. Russian olive is now the fourth most common riparian tree in the West (Friedman et al., 2005). In 1950, Russian olive was introduced to the Milk River in north-central Montana and by 1999 it had become the dominant tree species (Pearce and Smith, 2001). Competition from Russian olive and its effect altering flood disturbance regimes have been implicated in the decline in native cottonwood seedling recruitment along the Rio Grande in New Mexico (Knopf and Olsen, 1984; Howe and Knopf, 1991).
Because of its capacity to fix nitrogen, Russian olive invasions can also alter stream nutrient cycling dynamics (Mineau et al., 2011). Russian olive fruits are consumed by wildlife, including invasive non-native species forming a mutualistic relationship which further disperses seeds (Edwards et al., 2014). Russian olive displaces native plants, forming monocultures where wildlife diversity is less than in native plant communities (Brown, 1990; Stoleson and Finch, 2001; Wilson and Bernards, 2009). The presence of Russian olive trees in traditional duck nesting sites reduces nesting success (Gazda et al., 2002).
Mechanical removal of Russian olive by cutting down or pulling up trees without a herbicide treatment usually results in a thicker stand of Russian olive stems due to its prolific re-sprouting and suckering capacity (USDA, 2014). Herbicide applications to remaining stumps after cutting removal, referred to as a “cut-stump treatment”, have been shown to satisfactorily suppress regrowth of other invasive trees species with analogous basal re-sprouting abilities (DiTomaso and Kyser, 2007). However, cut-stump treatment efficacy can be dependent on the seasonal timing of treatments and the herbicide used (Ballard and Nowak, 2006). Most glyphosate product labels indicate the best time to apply cut-stump treatment is while the trees are actively growing. The challenge of this timing for farmers is that it is typically during their busiest time of year and interferes with production activities.
The objectives of this research were to 1) evaluate the efficacy of glyphosate, a readily available and cost effective herbicide, for cut-stump treatments on Russian olive regrowth and 2) identify seasonal effects on treatment efficacy.
Materials and methods
The research location chosen for this study was three separate subirrigated pastures, located within a one mile radius, located in Carbon County, Utah. Beginning in December 2009, trees were selected at random (N = 3), cut and treated each month of the year, through November 2012. The stumps were treated with Hi-Yield Super Concentrate Killzall, 41% glyphosate, at a rate of one milliliter per inch of trunk diameter. The label of the product states “flood the stump with undiluted product.” All stems of multi-stemmed plants were treated. Tree trunks ranged from three inches to 20 inches in diameter. Herbicide was metered with a livestock dosage syringe to insure that a consistent amount of herbicide was accurately applied to each stem. The herbicide was applied to the sap wood layer of the stump just beneath the bark, where translocation of photosynthates and water occurs (Patterson and Worwood, 2014). The heartwood of larger trees was not treated, as very little translocation occurs in that part of the tree. During the first and third year of the trial, additional trees (N = 3) were randomly selected, cut, and not treated with herbicide to serve as controls each month. Since stumps left untreated with herbicide re-sprouted vigorously, no control stumps where left during the second year in an attempt to reduce research activity negative impacts deemed unacceptable to the privately owned pasture.
Because Russian olives only partially killed will recover and continue to grow, control in this trial was evaluated as either dead or alive. Any regrowth indicated the tree was still alive, and thus not controlled. The final evaluation of treatment effects on regrowth occurred three years after the final treatment for all three years.
Control data were inferentially analyzed as percent mortality between herbicide treated and control stumps. Monthly control data for the first and third trial years were analyzed using the PROC GLM procedure while yearly control data were contrasted using the PROC TTEST Satterthwaite approximation due to unequal variance (SAS Studio).
Results
No significant differences were observed in percent mortality values between trial year one and three in 2009 to 2010 and 2012 (t = -1.48; df = 11; P = 0.166). Due to the overall similarity, control efficacy differences between months of treatment were analyzed together for year one and three. No significant differences in control were seen between months overall across both years (F = 0.91; df = 11; P = 0.559). Because untreated control stumps were not able to be left during trial year two in 2010, it could not be included in the analysis. The lack of degrees of freedom did not lend the data to analyses of both the main effects of trial year and month of treatment as well as any interactions that may have been present.
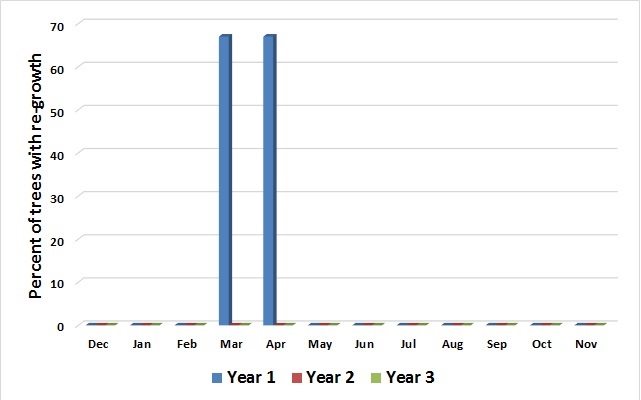
In every month during the three years of treatments, with the exception of stumps treated in March and April 2010, herbicide treated stumps were 100% controlled (Fig. 1). Sprouts from those stumps did not appear until two years after the stumps were treated, which indicates they arose from adventitious buds that perhaps developed after the glyphosate had become inactive. A final evaluation was done during the 2015 growing season, nearly three years after the last stumps were treated. The result was that 96% of the treated trees across the three treatment years showed no regrowth three years after treatment.
Figure 1. Percent of herbicide treated stumps with re-growth treated each month during three trial years.
Of the 72 control trees across all three treatment years, all except one re-sprouted vigorously from epicormic buds on the stumps (Fig. 2). No suckers emerged from the roots of treated or control stumps, which was thought to be due to the Russian olives in this location not having shallow or exposed roots.
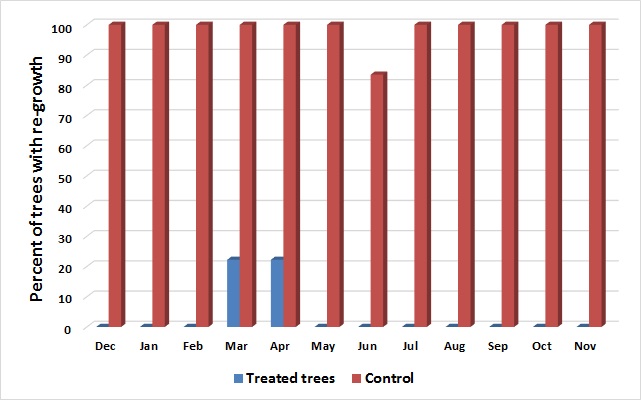
Figure 2. Re-growth comparison of percent of stumps treated with herbicide (shown in blue; N = 108) and control stumps without an herbicide treatment (shown in red; N = 72).
Conclusions
The cut-stump treatment method using glyphosate appears to be an effective year-round control option for Russian olive. It requires only a very small amount of herbicide, and poses minimal risk to non-target plants because the herbicide is applied directly to a specific area of the tree trunk.
Because glyphosate treatment during most of the year satisfactorily suppressed regrowth, cut-stump treatment has the added benefit of seasonal flexibility giving land managers a broad time interval to make effective removals. The encouraging results observed in this research warrant further large-scale examinations.
The cut-stump method is labor intensive. The most difficult part of the process is getting close enough to the trunk to cut the tree and administer the herbicide. A pruning saw and loppers are helpful to clear a path to the tree trunk. Protective clothing should be worn by anyone operating a chain saw, or who is working around Russian olives. Care should be taken during herbicide treatment since any cut stems left untreated will re-sprout. Follow-up treatments are needed for at least three years to treat sprouts and seedlings.
References
Ballard, B. D., and Nowak, C. A. (2006). Timing of cut-stump herbicide applications for killing hardwood trees on power line rights-of-way. Arboriculture & Urban Forestry, 32(3), 118-125.
Brown, C. R. (1990). Avian use of native and exotic riparian habitats on the Snake River, Idaho. Fort Collins, CO: Colorado State University. 60 p. Thesis.
DiTomaso, J. M., and Kyser, G.B. (2007). Control of Ailanthus altissima using stem herbicide application techniques. Arboriculture & Urban Forestry, 33(1), 55-63.Edwards, R. J., Clark, L.C. & Beck, K. G. (2014). Russian olive (Elaeagnus angustifolia) dispersal by European starlings (Sturnus vulgaris). Invasive Plant Science and Management, 7(3), 425-431.
Edwards, R.J., Clark, L.C., and Beck, K.G. (2014). Russian olive (Elaeagnus angustifolia) disperson by European starlings (Sturnus vulgaris). Invasiveive Plant Sciene and Management 7(3):427-751.
Friedman, J. M., Auble, G. T., Shafroth, P. B., Scott, M. L., Merigliano, M. F., Preehling, M. D., and Griffin, E. K. (2005). Dominance of non-native riparian trees in western USA. Biological Invasions, 7(4), 747-751.
Gazda, R., Meidinger, R., Ball, I., and Connelly, J. (2002). Relationships between Russian olive and duck nest success in Southeastern Idaho. Wildlife Society Bulletin, 30(2), 337-344.
Howe, W. H., and Knopf, F. L. (1991). On the imminent decline of Rio Grande cottonwoods in central New Mexico. The Southwestern Naturalist, 36(2), 218-224.
Knopf, F. L. and Olson, T. T. (1984). Naturalization of Russian-olive: implications to Rocky Mountain wildlife. Wildlife Society Bulletin 12, 289–298.
Mineau, M. M., Baxter, C. V., and Marcarelli, A. M. (2011). A non-native riparian tree (Elaeagnus angustifolia) changes nutrient dynamics in streams. Ecosystems, 14(3), 353-365.
Patterson, R. K., and Worwood, D. R. (2014). Russian Olive Control—Cut Stump Treatment. Utah State University Extension, Horticulture/Trees/2014-01pr.
Pearce, C. M., and Smith, D. G. (2001). Plains cottonwood's last stand: Can it survive invasion of Russian olive onto the Milk River Montana floodplain? Environmental Management, 28(5), 623-637.
Stoleson, S. H., and Finch, D. M. (2001). Breeding bird use of and nesting success in exotic Russian olive in New Mexico. The Wilson Bulletin, 113(4), 452-455.
USDA (2014). Field guide for managing Russian olive in the Southwest. TP-R3-16-24. Retrieved from https://www.fs.usda.gov/Internet/FSE_DOCUMENTS/stelprdb5410126.pdf.
Wilson, R. and Bernards, M. (2009). Weeds of Nebraska: Russian Olive. University of Nebraska, Lincoln [On-line]. http://ianrpubs.unl.edu/live/ec167/build/ec167.pdf.