Journal of the NACAA
ISSN 2158-9429
Volume 11, Issue 2 - December, 2018
Are Infusions from Aquatic Invasive Plants More Attractive to Egg-laying Mosquitoes than Infusions from Native Plants?
- Rector-Woods, P. , County Environmental Resource Mgmt Agent, Rutgers Cooperative Extension
Nitzsche, P.J., County Agent I, Rutgers Cooperative Extension
Mangiafico, S.S., County Environmental Resource Mgmt Agent II, Rutgers Cooperative Extension
Rosellini, M., Environmental Specialist, Morris County, Division of Mosquito Control
Ross, D., Environmental Stewardship Coordinator, Somerset County Park Commission
ABSTRACT
It has been speculated that some aquatic invasive plant species (AIS) may be especially attractive to egg-laying female mosquitoes or create highly productive mosquito habitat. From Aug. 20 to Sept. 10, 2015 and July 18 to Aug 29, 2016 we developed experiments in Morris County, New Jersey to examine the relationship between AIS and mosquito egg laying. Ovitraps and gravid traps were baited and deployed with infusions of invasive Hydrilla verticillata (hydrilla), invasive Pistia stratiotes (water lettuce), native Elodea canadensis (elodea), native Nymphaea sp. (water lily) and control (tap water). The ovitraps collected predominantly Aedes mosquito eggs and the gravid traps captured gravid Culex females. Overall, no significant difference in Aedes choice of treatment (chi square p =0.44 and chi square p =0.38 in 2015 and 2016, respectively) was found. In 2015 however, gravid traps baited with H. verticillata infusion captured significantly greater number of gravid Culex females over the sampling period than the control (chi square p<0.0001). In 2016, there was a significant difference in the proportions of cumulative gravid females collected across treatments (chi square p < 0.0001). Hydrilla verticillata had significantly greater cumulative proportion of Culex captures, followed by E. canadensis and the control. These results suggest that understanding the relationship between AIS and egg-laying habitat for Culex mosquitoes may be important for the management of the spread of AIS and mosquito-vector borne diseases.
Introduction
Mosquito borne diseases have increased in the U.S. population from 2004 to 2016 (Rosenberg et al., 2018). Mosquitoes vector several important human diseases including Zika virus (CDC, 2018), West Nile virus (Farajollahi et al., 2011), Chikungunya dengue (Grandadam et al., 2011), LaCrosse encephalitis viruses (Watts et al., 1972), and Eastern equine encephalitis (Mitchell et al., 1992).
There are at least 10 genera and 63 species of mosquitoes in New Jersey that have been identified (Farajollahi and Crans, 2012) and several are known to be human disease vectors. New Jersey ranks in the top 20% of states reporting cases of mosquito borne diseases in the U.S. and territories 2004–2016 (CDC, 2018). Two common species that may be vectors in suburban NJ where this study was conducted are Culex pipiens, the northern house mosquito and Aedes albopictus, the Asian tiger mosquito (Apperson et al., 2004). Culex species, both exotic, such as Cx. pipiens and native, such as Cx. restuans, are considered the most important vectors of West Nile Virus in the northeast (Johnson et al., 2015). Urban Culex are also associated with transmitting western encephalitis and St. Louis encephalitis to humans and heartworm to dogs (Jackman and Olson, 2002).
Ae. albopictus is the predominant container breeding urban Aedes species in north central New Jersey where the study was conducted, since it is too cold for Ae. aegypti, a tropical species, to overwinter. These species are considered important vectors of Zika virus (Ciota et al., 2017), as is Ae. vexans (O’Donnell et al., 2017). Ae. albopictus is also a vector for dengue virus (Chan et al., 1971), chikungunya virus (Grandadam et al., 2011) and also a vector for yellow fever (De Oliveira et al., 2003). Another exotic container-breeding mosquito, Aedes japonicus japonicus, occurs in the state but its population is usually relatively low especially during the summer months (Kaufman and Fonseca, 2014).
Mosquitoes require water during their immature life stages (Crans, 1994) and are often associated with stagnant, bacteria-filled environments. The growth of aquatic invasive plant species (AIS) can often create habitat with dense vegetation (Madsen, 2009) which slows or stagnates water through their rapid growth and by adding large amounts of decaying organic matter to waterbodies.
Gravid female mosquitoes use various cues to choose a breeding site; which are often species specific, although some species may use similar cues (Bentley and Day, 1989). Cues may be physical, chemical, biological or olfactory stimuli, the “smell” of larvae of the same or other mosquito species in the water, or predators, such as Gambusia, in the water can influence site selection (Gjullin et al., 1965; Gonzalez et al. 2016; Gubler, 1971).
Despite the importance of mosquitoes as disease vectors and their potential relationships with AIS, there are limited studies providing species-specific information. Studies to improve mosquito management for malaria prevention (Stone et al., 2018) or mosquito infestations due to wastewater treatment systems (Chandra et al., 2006) have provided information on relationships between specific aquatic plants and the oviposition or breeding of mosquito species. In NJ there has been little to none of this research conducted. Species-specific interactions between mosquito vectors and AIS is required to provide better information for management decisions.
The objective of this study was to determine if infusions from AIS or native plant species affect Aedes oviposition choices or attract gravid Culex females.
Aquatic Plants
Hydrilla verticillata (L.f.) Royle (hydrilla) is an extremely invasive aquatic plant that often form tangled mats at the water surface. The leaves form in a whorl around the stem, typically with five (5) leaves in a whorl (Rector and Nitzsche, 2015).
Elodea canadensis (elodea) is a plant native to NJ, in the same in the family as H. verticillata (Hydrocharitaceae). It has a similar growth pattern with leaves clustered in whorls around a stem, but characteristically in whorls of three leaves rather than the whorls of five leaves typical of H. verticillata. Although H. verticillata has been characterized as the “perfect weed” (Langeland, 1996), E. canadensis can grow to nuisance populations under certain conditions (Bowmer et al., 1995).
Pistia stratiotes L. (water lettuce) (family Arum) is a plant with floating leaves that is highly invasive in the U.S. When provided with the right conditions such as warm temperatures and high nutrients, P. stratiotes can increase rapidly (Fonkou et al., 2002).
Native Nymphaea (family Nymphaeaceae) has floating leaves and can form dense patches on top of the water. Water lilies are not typically considered a weed, but they are known to grow to high densities (Moore et al., 1994). Both Nymphaea and Pistia stratiotes can reproduce by rhizomes.
Materials and Methods
Plant Infusion of Water
Experiments were conducted during the summer of 2015 (20 Aug 2015 – 10 Sep 2015) and the summer of 2016 (18 Jul 2016 – 29 Aug 2016). Plant infusion treatments were made from two AIS, Hydrilla verticillata and Pistia stratiotes; and two native plants, Elodea canadensis and Nymphaea sp. The control for both years was tap water. In 2015, 240 g of plant material was placed in a heavy plastic 121 L covered container (20 in. L x 13 in. W x 16 in. H) with 5.7 L of tap water and placed in the shade with the lid on tightly for two weeks. After removal of infusion material for the second week of the experiment, additional tap water was added to each container (1.6 L / container) to allow sufficient volume to continue experiments for an additional week. In 2016, 534 g of plant material were placed in each of eight plastic garbage cans (121 L Rubbermaid heavy garbage can) with 10 L of tap water and placed in the shade with the lid on tightly for two weeks similar to Reiter (1991) and Trexler et al., (1998). Each plant infusion in each year was produced, aged and kept in one container for consistency until placed in one of the traps.
Mosquito Trapping
Two types of traps were used to collect mosquitoes and mosquito eggs. Aedes mosquito, such as Aedes albopictus and Aedes aegypti are adapted to small artificial containers. These diurnal mosquitoes are at least partially visually attracted. Black or blue ovitraps collected significantly more eggs than white or striped black and white ovitraps containers (Hoel et al., 2011). Alternatively, traps are set for Culex species at night. The CDC Gravid traps (Reiter 1983) are an improvement from earlier light traps. Gravid traps tend to utilize “stinky water” as an attractant. Several of the Culex species, including Culex pipiens, favor waters rich in organic matter. A study on the efficacy of the gravid traps for capturing females found 80-90% of females were alive in the morning, at least 95% were gravid and approximately 88 times more Culex mosquitoes were collected than resting site (storm culverts, other underground mosquito shelters) (Reiter et al., 1986). Gravid traps also seem to attract predominately Culex pipiens mosquitoes (Kesavaraju et al., 2011).
Ovitrap Experiment
Ovitraps, 0.473 L black hard plastic drinking cups, 8.9 x 6.4.12.8 cm (diameter of top x bottom x height) (Hoel et al., 2011) were filled with 320 ml of each plant infusion and control (20 replicates each). The inside of each cup was lined with foam board to provide oviposition substrate. Cups were placed on vacant, undeveloped property with sparse weeds/grass and sandy soil in 20-compartment cup plastic cup racks (sections 5 across x 4 down) racks (49.5 cm L X 49.5 cm W ) on the ground in a randomized grid.
Cups were emptied weekly, foam boards were collected, and eggs were counted. Cups were refilled with infusion, foam boards replaced and the location of cups within the grid was moved to prevent location bias. In 2016, the Nymphaea infusion was compromised with leeches and mosquito larvae; and, therefore, the Nymphaea treatment was not included in the 2016 study.
Gravid Trap Experiment
A second experiment was conducted concurrently using the infusion and control with gravid traps. Gravid traps (Box Gravid Mosquito Trap, Model 2800S, BioQuip) are rectangular bins that are partially filled with infusion water or control water. Female mosquitoes landing on the water to lay eggs are collected with a battery-operated fan that pulls the females into a net. In 2015, there were five trap treatments (Hydrilla verticillata, Elodea canadensis, Pistia stratiotes, Nymphaea and tap water), and in 2016 there were four trap treatments (H. verticillata, E. canadensis, P. stratiotes and tap water). The gravid traps were emptied every 1–4 days, batteries were exchanged and the female mosquitoes were counted and identified to species. Gravid traps will collect significantly more Cx. species (Reiter et al., 1986).
Statistical Analyses
Egg counts were summed for each year and treatment, and the proportion for each treatment was calculated. A chi-square goodness-of-fit test was performed to determine if the counts were statistically different across treatments (Zar, 2010; Agresti, 2013). A variant of Cramér's V for goodness-of-fit tests was calculated as a measure of effect size (Mangiafico, 2016). For a chi-square goodness-of-fit test with equal theoretical proportions, Cramér's V will vary from 0 to 1, where a value of 0 indicates no variation in proportions across categories, or no effect. Confidence intervals for the proportions for each treatment were calculated following the Sison and Glaz method for multinomial confidence intervals (Sison & Glaz, 1995). Treatments with non-overlapping 95% confidence intervals were considered statistically different. Counts for gravid females were treated similarly.
Statistical analyses were conducted in R (R Core Team, 2018), with package stats used for chi-square goodness-of-fit test, DescTools for confidence intervals for proportions, rcompanion for Cramer’s V , and ggplot2 for plots.
Sources of Materials
Hydrilla verticillata was retrieved from Alcyon Lake, Gloucester County, NJ in 2015 and 2016. E. canadensis was retrieved from Rutgers Snyder Research Farm, Hunterdon County, NJ in 2015 and 2016. Nymphaea sp. was purchased from Eli’s Aquarium, Morris County, NJ in 2015 and retrieved from Lake Musconetcong, NJ in 2016. P. stratiotes was purchased from Eli’s Aquarium, Morris County, NJ in 2015 and from Atlantis Aquarium, Morris County, NJ in 2016.
Results
Ovitraps
In 2015, 780 eggs were collected weekly over a period of three weeks 8/20/15 – 9/10/15, with a mean number of 260 eggs / week. No differences were found in the proportions of cumulative eggs collected across treatments in 2015 (chi-square p = 0.44), with the proportions of eggs collected across the five treatments ranging from 0.182 to 0.218 (Table 1). Cramér's V was 0.035, indicating a smaller than small effect size.
| Treatment |
Egg count |
Proportion of total |
Lower CI |
Upper CI |
Statistical Grouping |
|---|---|---|---|---|---|
| H. verticillata | 170 | 0.218 | 0.182 | 0.254 | n.s.† |
| E. canadensis | 164 | 0.210 | 0.174 | 0.246 | n.s. |
| Nymphaea sp. | 142 | 0.182 | 0.146 | 0.218 | n.s. |
| P. stratiotes | 159 | 0.203 | 0.168 | 0.240 | n.s. |
| Control | 145 | 0.186 | 0.150 | 0.221 | n.s. |
| Total | 780 | 1.000 |
†n.s., no treatments had non-overlapping 95% confidence intervals.
In 2016, the experiment ran for seven weeks, 7/18/16 – 8/29/16, and the total number of eggs collected was 2,029, for a mean of 290 eggs / week. No differences were found in the proportions of cumulative eggs collected across the treatments in 2016 (chi-square p = 0.38), with the proportions of eggs collected across treatments ranging from 0.240 to 0.264 (Table 2). Cramér's V was 0.023, indicating a smaller than small effect size.
| Treatment |
Egg count |
Proportions of total |
Lower CI |
Upper CI |
Statistical grouping |
|---|---|---|---|---|---|
| H. verticillata | 535 | 0.264 | 0.241 | 0.287 | n.s.† |
| E. canadesis | 517 | 0.255 | 0.232 | 0.279 | n.s. |
| P. stratiotes | 487 | 0.240 | 0.217 | 0.264 | n.s. |
| Control | 490 | 0.241 | 0.218 | 0.265 | n.s. |
| Total | 2029 | 1.000 |
†n.s., no treatments had non-overlapping 95% confidence intervals.
Gravid Female Traps
In 2015, during 19 sampling events, 4,789 gravid females were collected for a mean of 252/sampling event. Significant differences were found in the proportions of cumulative gravid females collected across treatments in 2015 (chi-square p < 0.0001). Cramér's V was 0.310, indicating a large effect size. The H. verticillata treatment had the statistically greatest cumulative gravid female proportion, followed by Nymphaea sp. and P. stratiotes (Table 3, Figure 1). All plant infusion treatments had greater proportions than did the control.
| Treatment | Gravid female count | Proportion of total | Lower CI | Upper CI | Statistical grouping | |
|---|---|---|---|---|---|---|
| H. verticillata | 1832 | 0.382 | 0.367 |
0.398 |
a† | |
| E. canadensis | 556 | 0.116 | 0.101 | 0.131 | c | |
|
1168 | 0.244 | 0.229 | 0.259 | b | |
| Nymphaea sp. | 1143 | 0.239 | 0.223 | 0.254 | b | |
| Control | 190 | 0.019 | 0.004 | 0.034 | d | |
| Total | 4789 | 1.000 |
†n.s. Values sharing a letter are not statistically different.
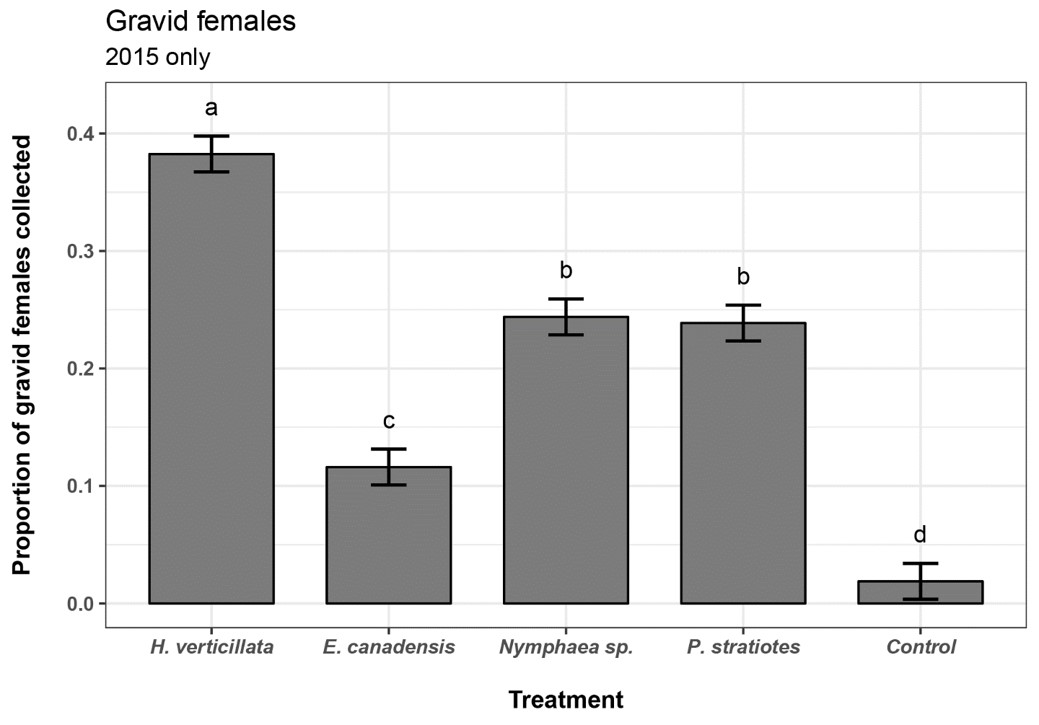
Figure 1. Proportion of counts for cumulative gravid female mosquitoes in 2015. Error bars represent the 95% multinomial confidence interval by Sison and Glaz method. Values sharing a letter are not statistically different. Values with non-overlapping 95% confidence intervals were considered statistically different.
Twenty-six sampling events were conducted in 2016, and a total of 2,536 gravid females were collected, for a mean of 98 gravid females/sampling event. Significant differences were found in the proportions of cumulative gravid females collected across treatments in 2016 (chi-square p < 0.0001). Cramér's V was 0.184, a medium effect size. The H. verticillata treatment had the statistically greatest cumulative gravid female proportion, followed by Control and E. canadensis (Table 4, Figure 2). Only the Hydrilla verticillata treatment had a statistically higher proportion of gravid females than the control.
| Treatment | Gravid female count | Proportion of total | Lower CI | Upper CI | Statistical grouping |
| H. verticillata | 933 | 0.368 | 0.347 | 0.389 | a† |
| E. canadensis | 585 | 0.231 | 0.210 | 0.252 | b |
| P. stratiotes | 366 | 0.144 | 0.123 | 0.165 | c |
| Control | 652 | 0.257 | 0.236 | 0.278 | d |
| Total | 2536 | 1.000 |
†values sharing a letter are not statistically different.
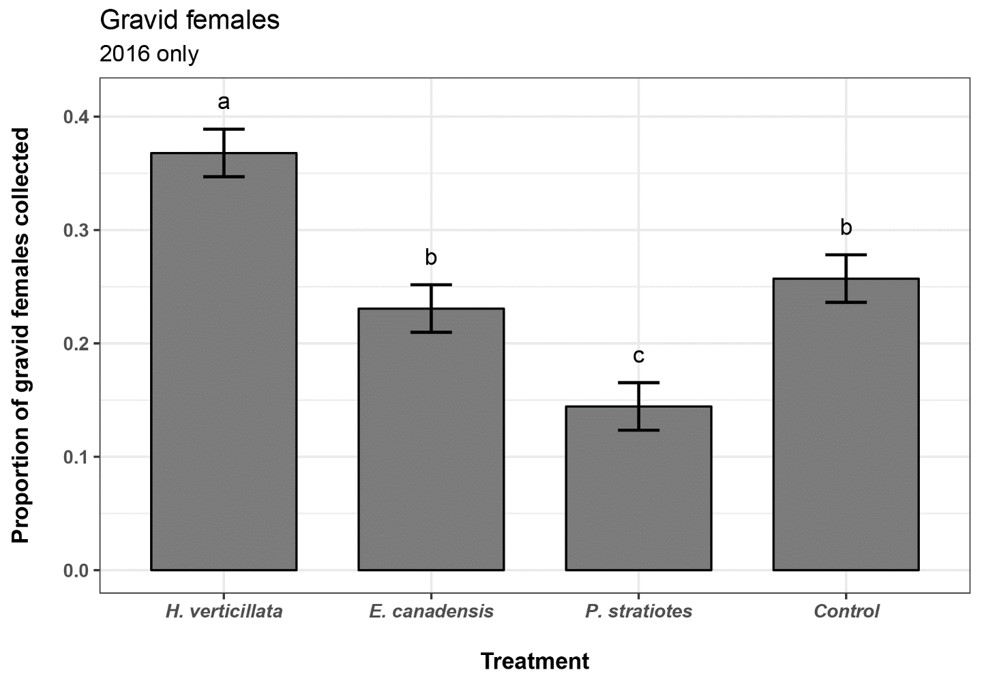
Figure 2. Proportion of counts for cumulative gravid female mosquitoes in 2016. Error bars represent the 95% multinomial confidence interval by Sison and Glaz method. Values sharing a letter are not statistically different. Values with non-overlapping 95% confidence intervals were considered statistically different.
Discussion
Aedes oviposition
In NJ it is Aedes mosquitoes that lay eggs in containers on substrates (D. M. Fonseca personal communication. August 29, 2018). There are several potential Aedes species that might lay eggs in ovitraps in NJ including; Ae. albopictus, Ae. japonicas, Ae. triseriarus, or Ae. atropalpus (D.M. Fonseca personal communication August 13, 2018), but in the hottest part of the summer, when the experiments were conducted, the eggs will likely be predominately Ae. albopictus (Fonseca et al., 2013).
In this study, Aedes did not show a preference in oviposition among plant-infused treatments or for plant-infused treatments over the control (Table 1, Table 2). In other studies, Ae. albopictus has been shown to have a preference for infusions of white oak (Ponnusamy et al., 2010; Trexlar et al., 1998). Individually, infusions of bamboo, Bermuda hay or hackberry were attractive to Ae. albopictus females compared to a control (well water) (Ponnusamy et al., 2010). On the other hand, female Ae. albopictus visits to infusions of live oak, red maple, pecan or panic grass were not significantly different than visits to the control, indicating a lack of preference to these plant infusions (Ponnusamy et al., 2010).
Angerilli (1980) found that water with extracts from Elodea canadensis or Nymphaea tuberosa were significantly less attractive to Ae. aegypti for oviposition than distilled water under laboratory conditions. As described above there was no significant difference, and we did not see a significant aversion to these plant infusions with the oviposition treatments.
While the lack of differences in oviposition among treatments may be the result of an actual lack of preference of this mosquito for these treatments, another explanation may be skip oviposition. This is an environmental response exhibited by container mosquitoes such as Ae. albopictus and Ae. aegypti, where the gravid female will lay eggs in many potential sites to both reduce intraspecific competition and spread the risk. Skip depositing has been found to occur when females are exposed to low quality habitat, the behavior increasing overall fitness (Davis et al., 2015). Future studies may consider an experiment with self-marking ovitraps more conducive to Aedes ovipositing to avoid the confounding effects of skip oviposition (Davis et al., 2016).
Culex oviposition
The H. verticillata treatment attracted a significantly greater proportion of gravid females compared with other plant-infused treatments, including E. canadensis, or tap water controls (Tables 3 and 4, Figures 1 and 2). Other plant-infused treatments were less consistent. For example, E. canadensis, Nymphaea sp., and P. stratiotes attracted a greater proportion of gravid females in 2015 than did the control, but in 2016 no plant-infused treatments other than H. verticillata were greater than the tap water control. This could be related to a change in the attributes of the infusions across years or other seasonal factors.
The results for P. stratiotes were inconsistent across years, having a higher proportion of gravid females captured than E. canadensis or the control in 2015, but having a lower proportion than either of these in 2016. Pistia stratiotes has been linked to Mansoides mosquito species, including Mansonia annulifera, a vector of Human brugian filariasis, a disease affecting 13 million people (Chandra et al., 2006). Preference for P. stratiotes (47.2%) and Eichhornia crassipes (41.5%) by Mansonides was significantly higher than for samples with Azolla pinnata, Mimosa pudica or the control (Chandra et al., 2006). A new alternative wastewater treatment plant in Cameroon had many advantages but mosquitoes were a disadvantage that was investigated. Studies found highest number of mosquitoes were Mansonia larvae attached to the P. stratiotes roots with the second greatest relative abundance being Culex mosquitoes (Cx. quinquefasciatus,, Cx. decens and Cx. tigripes) free living, not attached to the P. stratiotes roots (Kengne et al., 2003). Few mosquitoes known to be disease vectors were found in this study.
Other studies have also found Culex species to have preferences for specific aquatic plants. In a study in Australia of Cx. annulirostris Skuse, there was a significant difference in the number of gravid females laying their egg rafts in buckets with Salvinia adnata (giant salvinia) compared to Eichhornia crassipes (common water hyacinth) and Cyperus prolifer (dwarf papyrus) (Webb et al. 2012). Cx. quinquefasciatus, the southern house mosquito, did not exhibit a significant egg laying preference in buckets with either E. crassipes, Cyperus haspens (Dwarf papyrus), or Salvinia molesta (giant salvinia) and aged tap water located in a plastic tent (Webb et al., 2012). All three plants are non-native to Australia, and S. adnata and E. crassipes are on Australia’s List of Weeds of National Significance.
Hydrilla verticillata was found to provide habitat for Culex mosquitoes in another Australian study (Hearnden and Kay, 1997). Three separate habitats were surveyed for immature mosquitoes; shoreline water with vegetation, three Hydrilla verticillata beds 50—100 m offshore and a third sampling site consisting of muddy holes, watercourses or natural pools located around a reservoir. Anopheles was dominant (43.7%) and Cx. annulirostris had the second largest mosquito population (29.9%). There were significantly higher populations of immature mosquitoes in the three emergent H. verticillata beds than the other sites, and survivorship at the emergent H. verticillata beds was greater than for the shoreline or the third sampling site (Hearnden and Kay 1997).
The attraction to specific aquatic plants by certain mosquitoes was connected to very specific biological chemicals released by the plants (Sérandour et al., 2008). These included uracil, thymine, glycerol, and the pyrimidines uridine and thymidine in specific concentrations, and were related to root cells breaking down in the water. For Coquillettidia species larvae, this release of five specific chemicals provides a chemical call to plant species with the necessary root system to penetrate and capture oxygen from the arenchyma.
Conclusion
The spread of AIS in the environment is likely to continue as well as the problem of human diseases vectored by mosquitoes. This study found a potential relationship between H. verticillata and attraction of egg-laying Culex females as compared to the control, P. stratiotes, E. canadensis, and Nymphaea, but did not find a significant relationship between Aedes oviposition and the plant species investigated. Understanding the interactions between mosquito vectors and AIS is important in making management decisions and the proper marshalling of resources to address the spread of AIS as well as mosquito vector borne diseases.
Literature Cited
Agresti, A. (2013). Categorical Data Analysis, 3rd ed. Hoboken, NJ. John Wiley and Sons.
Angerilli, N.P.D. (1980). Influences of extracts of freshwater vegetation on the survival and oviposition of Aedes aegypti (Diptera: Culicidae). The Canadian Entomoligist,12:1249-1252.
Apperson, C.S., Hassan, H.K., Harrison, B.A., Savage, H.M., Aspen, S.E., g, A., Crans, W., Daniels, T.J., Falco, R.C., Benedict, M., McMillen, L., and Unnasch, T.R. (2004). Host feeding patterns of established and potential mosquito vectors of West Nile virus in the eastern United States. Vector Borne Zoonotic Diseases, 4(1):71–82.
Bentley, M.D. and Day, J.F. (1989). Chemical ecology and behavioral aspects of mosquito oviposition. Annual Review of Entomology, 34:401–421.
Bowmer, K.H., Jacobs, S.W.L. and Sainty, G.R. (1995). Identification, biology and management of Elodea canadensis, Hydrocharitaceae. Journal of Aquat. Plant Manage., 33:13–19.
CDC Center for Disease Control and Prevention. (2018). Illnesses on the rise from mosquito, tick and flea bites. CDC Vital Signs May 2018. Retrieved from https://www.cdc.gov/vitalsigns/pdf/vs-0518-vector-borne-H.pdf Centers for Disease Control and Prevention National Center for Emerging and Zoonotic Infectious Diseases, 1600 Clifton Road NE, Atlanta, GA 30333.
Chan, Y.C., Ho, B.C. and Chan, K.L. (1971). Aedes aegypti (L.) and Aedes albopictus (Skuse) in Singapore City 5. Observations in relation to Dengue Haemorrhagic Fever. Bull. Wld Hlth Org., 44:651-658.
Chandra, G., Ghosh, A., Biswas, D., and Chatterjee, S.N. (2006). Host plant preference of Mansonia mosquitoes. Journal of Aquat. Plant Manage., 44:142–144.
Ciota, A.T., Bialosuknia, S.M., Zink, S.D., Brecher, M., Ehrbar, D.J., Morrissette, M.N., and Kramer. L.D. (2017). Effects of Zika virus strain and Aedes mosquito species on vector competence. Emerging Infectious Diseases 23(7):1110-1117.
Crans, W.J. (1994). Controlling mosquitoes around the home. Fact sheet 780. Rutgers Cooperative Extension, Rutgers New Jersey Agricultural Experiment Station, New Brunswick.
Davis, T.J., Kaufman, P.E., Hogsette, J.A. and Kline, D.L. (2015). The effects of larval habitat quality on Ae. albopictus skip oviposition. Journal of the American Mosquito Control Association, 31(4):321–328.
Davis, T.J., Kaufman, P.E., Tatem, A.J., Hogsette, J.A. and Kline, D.L. (2016). Development and evaluation of an attractive self-marking ovitrap to measure dispersal and determine skip oviposition in Aedes albopictus (Diptera: Culicidea) field populations. Journal of Medical Entomology, 53(1):31-38.
De Oliveira, R.L., Vazeille, M., DeFilippis, A.M. B., and Failloux, A-B. (2003). Large genetic differentiation and low variation in vector competence for dengue and yellow fever viruses of Aedes albopictus from Brazil, the United States, and the Cayman Islands. American Journal of Tropical Medical Hygiene. 69(1):105-118.
Farajollahi, A., Fonseca, D.M., Kramer, L.D., and Kilpatrick A.M. (2011). “Bird biting” mosquitoes and human disease: A review of the role of Cx. pipiens complex mosquites in epidemiology. Infection, Genetics and Evolution, 11:1577–1585.
Farajollahi, A., and Crans, S.C. (2012). A checklist of the mosquitoes of New Jersey with notes on established invasive species. Journal of the American Mosquito Control Association, 28(3): 237-239.
Fonkou, T., Agendia, F., Kengne, I., Akea, A., and Nya, J. (2002). Potentials of water lettuce (Pistia stratiotes) in domestic sewage treatment with macrophyte lagoon systems in Cameroon. Proceedings of the International Symposia on Environmental Pollution Control and Water Management, Tunis. Pp 702–714.
Fonseca, D.M., Unlu, I., Crepeau, T., Farajollahi, A. Healy, S.P., Bartlett-Healy, K., Strickman, D., Gaugler, R., Hamilton, G., Kline, D., and Clark, G.G. (2013). Area-wide management of Ae. albopictus. Part 2: Gauging the efficacy of traditional integrated pest control measures against urban container mosquitoes. Pest Manag Sci., 69:1351–1361.
Gonzalez, P.V., Gonzalez Audino, P.A. and Masuh, H.M. (2016). Oviposition Behavior in Ae. aegypti and Ae. alboptictus (Diptera: Culicidae) in response to the presence of heterospecific and conspecific larvae. Journal of Medical Entomology, 53(2):269–272.
Gjullin, C.M., Johnsen, J.O, Plapp, F.W. Jr. (1965). The effect of odors released by various waters on the oviposition sites selected by two species of Cx. Mosquito News. 25(3):268–271.
Grandadam, M., Caro, V., Plumet, S., Thiberge, J.M., Souares, Y., Failloux, A-B, Tolou, H.J., Budelot, M. Cosserat, D., Leparc-Goffart, I., and Despres, P. (2011). Chikungunya virus, southeastern France. Emerging Infectious Diseases, 17(5):910-913.
Gubler, D J. (1971). Studies on the comparative oviposition behavior of Ae. albopictus and Ae. ploynesiensis. Journal of Med. Entomology. 8:675–682.
Hearnden, M.N. and Kay, B.H. (1997). Importance of Hydrilla verticillata (Hydrocharitaceae) as habitat for immature mosquitoes at the Ross River Reservoir, Australia. Journal of the American Mosquito Control Association, 13(2):164 –170.
Hoel, D.F., Obenauer, P.J., Clark, M., Smith, R., Hughes, T.H., Larson, R.T., Diclaro, J.W. and S.A. Allan. (2011). Efficacy of ovitraps colors and patterns for attracting Ae. albopictus at suburban field sites inn North-Central Florida. Journal of the American Mosquito Control Association, 27(3): 245–251.
Jackman, J.A. and Olson, J.K. (2002). Mosquitoes and the disease they transmit. Texas A and M Fact Sheet B-6119. Texas A and M Publications http://terry.agrilife.org/files/2011/09/mosquitos-and-diseases.pdf Retrieved Aug. 8, 2018.
Johnson, B.J., Robson, M.G., and Fonseca, D.M. (2015). Unexpected spatiotemporal abundance of infected Culex restuans suggest a greater role as a West Nile virus vector for this native species. Infection, Genetics and Evolution, 31:40-47.
Kaufman, M.G. and Fonseca, D.M. (2014). Invasion Biology of Aedes japonicas japonicas (Diptera: Culicidae). Annu. Rev. Entomol., 59:31-49.
Kengne, I.M., Brissaud, F., Akoa, A., Eteme, R.A., Nya, J., Ndikefor, A., and Fonkou, T. (2003). Mosquito development in a macrophyte-based wastewater treatment plant in Cameroon (Central Africa). Ecological Engineering, 21:53–61.
Kesavaraju, B., Kiyoguchi, D. & Dickson, S. (2011). Efficacy of gravid traps in trapping Cx. pipiens. Journal of the American Mosquito Control Association, 27(3):320–322.
Langeland, K.A. (1996). Hydrilla verticillata (L.F.) Royle (Hydrocharitaceae), “The Perfect aquatic weed”. Castanea 61(3):293-304.
Madsen, J.D. (2009). Chapter 1: Impact of invasive aquatic plants on aquatic biology. pp. 1–8. In Biology and control of aquatic plants: a best management practices handbook (Gettys, L.A., Haller, W.T. and Bellaus, M.) (Eds.). Aquatic Restoration Foundation, Marietta GA. 210 pages.
Mangiafico, S.S. (2016). Goodness-of-Fit Tests for Nominal Variables. In Mangiafico, S.S., Summary and Analysis of Extension Program Evaluation in R. http://rcompanion.org/handbook/H_03.html.
Mitchell, C.J., Niebylski, M.L., Smith, G.C., Karabatsos, Martin, D., Mutebi, J.P., Craig, G.B., Jr., and Mahler, M.J. (1992). Isolation of Eastern equine encephalitis virus from Aedes albopictus in Florida. Science, 257:526-527.
Moore, B.C., Funk, W.H. and Anderson, E. (1994). Water quality, fishery, and biologic characteristics in a shallow, eutrophic lake with dense macrophyte populations. Lake and Reservoir Management, 8(2):175-188.
O’Donnell, K.L., Bixby, M.A., Morin, K.J., Bradley, D.S., and Vaughan, J.A. (2017). Potential of a northern population of Aedes vexans (Diptera: Culicidae) to transmit Zika Virus. Journal of Medical Entomology, 54(5):1354-1359.
Ponnusamy, L., Xu, N., Boroczky, K., Wesson, D.M., Ayyash, L.A., Schal, C. and Apperson, C.S. (2010). Oviposition responses of the mosquitoes Ae. Aegypti and Ae. albopictus to experimental plant infusions in laboratory bioassays. Journal of Chemical Ecology, 36(7):709–719.
R Core Team. (2018). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/.
Rector, P. and P.J. Nitzsche. (2015). Pond and lake management Part VII. Aquatic invasive species: Hydrilla (Hydrilla verticulata) - Prevention and management. Rutgers Cooperative Extension Fact Sheet No. FS . New Brunswick, NJ. 4pp.
Reiter, P. (1983). A portable, battery-powered trap for collecting gravid Culex mosquitoes. Mosquito News, 43:496-498.
Reiter, P., Jakob, W.L., Francy, D.B. and Mullenix, J.B. (1986). Evaluation of the CDC gravid trap for the surveillance of St. Louis encephalitis vectors in Memphis, Tennessee. Journal of American Mosquito Control Association, 2(2):209–212.
Reiter, P.M. (1991). Enhancement of the CDC ovitraps with hay infusion for daily monitoring of Ae. aegypti populations. Journal of American Mosquito Control Association, 7:52–55.
Rosenberg R., Lindsey N.P., Fischer M., Gregory, C.J., Hinckley, A.F., Mead, P.S., Paz-Bailey, G., Petersen, L.R. (2018). Vital signs: Trends in reported vectorborne disease cases — United States and Territories, 2004–2016. CDC Centers for Disease Control and Preventon Report 2018;67:496–501. https://www.cdc.gov/mmwr/volumes/67/wr/mm6717e1.htm#suggestedcitation .
Sison, C.P., and Glaz, J. (1995). Simultaneous confidence intervals and sample size determination for multinomial proportions. Journal of the American Statistical Association, 90(429):366–369.
Sérandour, J., Reynaud, S., Willison, J., Patouraux, J., Gaude, T., Ravanel, P., Lempériere, G. and M. Raveton (2008). Ubiquitous water-soluble molecules in aquatic plant exudates determine specific insect attraction. PloS ONE 3(10): e3350. https://doi.org/10.1371/journal.pone.0003350.
Stone, C.M., Witt, A.B.R., Walsh, G.C., Foster, W.A. and Murphy, S.T. (2018). Would the control of invasive alien plants reduce malaria transmission? A review. Parasites and Vectors, 11:76–94.
Trexler, J.D., Apperson, C.S. and Schal, C. (1998). Laboratory and field evaluations of oviposition responses of Ae. albopictus and Ae. triseriatus (Diptera: Culcidae) to oak leaf infusions. Journal of Medical Entomology, 35(6):967–976.
Watts, D.M., Morris, C.D., Wright, R.E., DeFoliart, G.R., and Hanson, R.P. (1972). Transmission of lacrosse virus (California encephalitis group) by the mosquito Aedes triseriatus. Journal of Medical Entomology, 9(2):125-127.
Webb, C.E., Ironside, A. and Mansfield, S. (2012). A comparison of oviposition preference in the presence of three aquatic plants by the mosquitoes Cx. annulirostris Skuse and Cx. quinquefasciatus Say (Culicidae: Diptera) in laboratory tests. Gen. Appl. Ent., 41:21–26.
Zar, J.H. (2010). Biostatistical Analysis, 5th ed. Upper Saddle River, NJ. Prentice Hall.
Acknowledgements
This research was partially funded by the Agricultural Agents Association of New Jersey (AAANJ).
The authors thank Prof. Dina Fonseca, Rutgers University, Department of Entomology, Director of the Center for Vector Biology and Kristian McMorland, Supervisor Morris County Division of Mosquito Control for review of this paper.
