Journal of the NACAA
ISSN 2158-9429
Volume 11, Issue 2 - December, 2018
Garden Myth Busting for Extension Educators: Reviewing the Literature on Landscape Trees
- Chalker-Scott, L. , Extension Specialist And Associate Professor, Washington State University
Downer, A.J., Farm Advisor, University of California
ABSTRACT
Horticultural myths, found extensively in print and online resources, are passed along by uninformed gardeners, nursery staff, and landscape professionals. Occasionally myths are so compelling that they make their way into Extension publications, used by Master Gardeners as educational resources. In this article we deconstruct seven widespread gardening myths by way of reviewing research-based literature. We also provide scientifically sound alternatives to these gardening practices and products. Our hope is to arm Extension educators with the educational resources necessary to battle misinformation that ranges from the merely useless to that which is actively damaging to soils, plants, and the surrounding environment.
Introduction
Home gardeners and landscape professionals are a rapidly growing audience for extension educators as they seek science-based information to support their activities. However, many are not familiar with current research and cannot assess whether the information they find in print, on the internet, or through social media is accurate. In addition, some products and practices are meant for agricultural production, not for maintaining home gardens and landscapes. The combination of misinformation and misapplied information means that this audience risks damaging their plants and soils through overuse of fertilizers, misuse of pesticides, and poor management practices.
The field of urban horticulture, including arboriculture, is expanding with new insights about plants and soils in residential and public landscapes. However, there are few Extension educators who have an academic background in environmental horticulture and may be as confused as the public about what constitutes sound, science-based recommendations.
The authors of this article are state Cooperative Extension educators and researchers with many years of experience in translating science for use by home gardeners and landscape professionals. Our goal is to assist other Extension educators by providing reliable information for them to share with the gardening and landscaping public.
The purposes of this literature review article are:
- to identify some common beliefs homeowners and landscape professionals have about managing landscape plants and soils;
- to provide a brief, science-based explanation on why these beliefs are not accurate;
- to provide links to published, peer-reviewed information that supports the explanation and can be distributed to clientele; and
- to suggest strategies based on current and relevant applied plant and soil sciences for managed landscapes.
Myth Deconstruction
Myth #1: “Native trees and shrubs are superior to introduced species for wildlife habitat”
Many landscapes are overrun by exotic species deliberately introduced for ornamental or revegetation purposes. Research documents how exotic, invasive species can outcompete native plants and decrease the ecological and aesthetic value of landscapes (Figure 1; Reichard and White, 2001). As a result, homeowner associations, communities and entire cities have adopted “native only” planting specifications. Such policies have the unfortunate side effect of eliminating any exotic, noninvasive species from planting consideration.
Figure 1. English ivy (Hedera helix) has invaded this remnant forest in Seattle, having escaped from a residential landscape.
The rationale for native-only policies has centered on the belief that native plants have coevolved with native animals and are uniquely suited to providing food and shelter for them. What believers fail to consider is that urbanized landscapes are no longer natural (Figure 2). Construction activities include:
- vegetation removal;
- soil excavation and compaction;
- infrastructure development; and
- building development.

Figure 2. While native to the Pacific Northwest, Arbutus menziesii performs poorly in urban settings.
Landscapes planted after construction face a radically changed environment: many native species cannot tolerate the alterations in light, water, and temperature that come with urbanization. As a result, many native-only plantings suffer severe environmental stress and mortality.
Rather than limiting planting palettes to native species (which may be poorly adapted to urban conditions), noninvasive exotic species adapted to these harsher conditions should be considered. A robust body of research supports the use of introduced trees and shrubs in residential and public landscapes in addition to appropriate native species (Chalker-Scott, 2018; 2015b). Not only do they tolerate urban conditions, they provide habitat for wildlife just as effectively as native trees and shrubs.
Additionally, there are other activities that will improve wildlife biodiversity in managed landscapes. These include:
- reducing lawns using a mixture of groundcovers;
- providing a permanent water feature;
- protecting soils with a coarse woody mulch, such as arborist wood chips; and
- reducing or eliminating the use of pesticides.
Myth #2: “Many trees are difficult to transplant because of their long taproot”
The landscape below ground is little understood by gardeners and landscape professionals, whose perceptions are shaped by “common sense” assumptions. Everyone has seen a germinating seed before, including the long, unbranched taproot that develops before significant above-ground growth occurs. Once leaves appear, little thought is given to what root systems are doing. Many people believe that the taproot continues to grow, resembling a giant carrot especially in excurrent trees (those with holiday tree forms). They also believe that decurrent trees (those with classic shade tree forms) develop a more branched root structure, much like a giant broccoli cluster. In fact, popular renditions of root system show startling symmetries between crown and root structure. We like symmetry and this perception of mirror-imaged roots and crowns is appealing. It’s not surprising that professional recommendations for irrigation and fertilizer application are based on the belief that the “dripline” (the outer edge of the crown from which rainwater drips) encompasses the majority of the tree’s roots.
In fact, the taproot structure in nearly all trees is a juvenile feature with specific functions important to young trees (Chalker-Scott, 2015a):
- it anchors the young tree;
- it creates a vertical structure for lateral root development;
- it stores sugars transferred from the leaves; and
- it serves as the conduit for water and nutrients to the trunk and canopy.
What it does not do is absorb significant amounts of water and nutrients. This function is provided by root hairs, which are fine, thread-like structures found at the growing tips of young roots. The taproot itself only has one growing point.
What determines where roots will grow in the soil?
Roots require three things to survive underground: water, nutrients, and oxygen. When one or more of these are missing from the root environment (also called the rhizosphere), root growth stops. For taproots, downward growth usually slows as oxygen becomes more limiting with soil depth. As the young tree matures, more lateral roots explore soil resources in the top 12-18” of soil (Figure 3).
Figure 3. Mature landscape trees have no tap root.
Highly drained, sandy soils can have tree roots that extend far below the surface, because they are well-aerated and hydrated. This scenario is unlikely in most developed areas, where construction activities compact the soil, compressing pore space and driving oxygen out. Urban soils and slowly draining wetland soils do not have sufficient oxygen to allow for deep root establishment.
Thus, as a tree matures, its lateral root spread becomes greater. It is currently estimated that a tree’s functional root system extends two to three times the diameter of the crown at its widest point. These are the roots that are supplying the tree with water and nutrients. The juvenile taproot is subsumed by the rapidly developing lateral root system (Chalker-Scott, 2015a).
Myth 3: “Mycorrhizal inoculants should be added to planting holes when installing woody ornamentals in landscapes”
Beneficial fungi called mycorrhizae associate with roots of woody plants in a mutualistic relationship that benefits each partner. Decades of research have shown how plants inoculated with mycorrhizae grow faster and larger than those without these fungal partners (Figure 4; Carpio et al., 2005). This knowledge has prompted entrepreneurs to market mycorrhizal inoculant products as planting amendments, claiming they will ensure establishment of landscape plants. Mycorrhizal inoculants are often seen in container (potting) media, organic fertilizers, or sold separately as growth promoting products, but their efficacy is often unproven, especially in landscape situations (Chalker-Scott, 2017).

Figure 4. Mycorrhizal fungi are essential to healthy plants and soil.
Misconceptions about mycorrhizal products are common:
-
My soil is “poor” so I have to add the mycorrhizae to my garden. Most soils are already inoculated with mycorrhizal fungi, so they do not need to be added. Plants will form mycorrhizal associations in most soils without additional inoculant. Soils where mycorrhizal inoculants have been effective in promoting plant growth responses are extremely disturbed or toxic, such as mine spoils or severely “cut” sites where all surface soil is removed and plants are being grown in subsoils.
-
Mycorrhizal inoculants will support my aging or dying tree. While research shows that mycorrhizae play a role in plant defense from pathogens (Linderman, 1988), there is no evidence to suggest that inoculants can provide additional benefit to previously inoculated plants.
-
Mycorrhizae will promote growth of my established trees. There is no published work to indicate inoculation of established trees will promote their growth; Appleton et al. (2003) reported no effect from this application.
-
The mycorrhizal inoculant contains viable spores. Biological products have limited shelf life. Research on commonly available inoculants showed that over 50% of the products available to consumers were not viable (Corkidi et al., 2003). Mycorrhizal fungi have naturally low viability of their spores so if a product does not have hyphal fragments included, it may not be viable.
-
If I inoculate with mycorrhizae I don’t need to fertilize my plants. Mycorrhizae aid in nutrient uptake (especially phosphorus), but they do not cure nutrient deficiencies, especially when the soil is deficient in those minerals. Research indicates that mycorrhizae can enhance uptake of normally available soil nutrients, but the soil has to contain them in the first place (Corkidi et al., 2005).
-
If I add mycorrhizae I will bring life to my soil. If a soil is devoid of microbial activity, it is likely an unsuitable soil for mycorrhizae to grow in. Just like plants, mycorrhizal fungi need good soil conditions in which to grow. Compacted, flooded, or contaminated soils are also harmful to these fungi, so they will not cure a toxic or otherwise non-arable soil.
Myth 4: “The crowns of transplanted trees should be pruned to compensate for root loss”
Earlier we mentioned that humans like symmetry. This bias seeps into the practice of pruning the crowns of trees and shrubs during transplanting. There is a widespread belief that the crown size should mirror the roots to avoid straining the root system for water uptake. Initially this practice does reduce water usage, but soon the plant responds with new shoots and leaves, requiring not only more water but nutritional resources for their development.
Understanding how plants work is useful in explaining this response (Bayala et al., 2004; Jones et al., 1998; Martin et al., 2010):
- cutting branches removes growth-regulating auxins and allows dormant buds below these cuts to grow;
- new branches and their leaves require nitrogen and other essential nutrients for their development;
- expanding leaves require high levels of water to maximize leaf size;
- directing water and nutrients to the crown reduces their availability to roots;
- root growth and establishment is reduced without sufficient water and nutrients;
- root growth is further inhibited by the lack of auxins, which stimulate new root development, and
- poor root systems are unable to take up sufficient water to support the crown.
Gardeners see the tops of their transplanted trees growing and mistakenly think the tree is healthy, only to see the tree suffer or die in subsequent months. Instead, the crown should be left intact (Figure 5). Again, understanding what is happening below ground is important (Chalker-Scott, 2015a):
- the tree’s first response after transplanting will be to grow new roots to establish into the surrounding soil;
- water and resources are directed to the roots, and above-ground growth will slow or stop during this time;
- flowers or leaves on transplanted trees may wilt or die, particularly if they are young or conditions are hot and dry;
- once the roots have established, water and resources will be directed to dormant buds; and
- the emerging shoots and leaves are an indicator that roots are well established into the soil.

Figure 5. Compensatory pruning is not recommended for newly transplanted trees and shrubs.
Myth 5: “Pruning cuts and other wounds should be sealed to prevent disease”
For many years, gardeners and tree care professionals covered wounds and pruning cuts with sealants such as asphalt emulsions or resin-based products. Sealing was recommended because treated wounds were thought to cover over more quickly than unpainted cuts, and that vulnerable tissues were isolated from decay fungi. Both of these suppositions were later disproven, and professionals soon abandoned an unnecessary and harmful practice (Shigo and Shortle, 1983). Unfortunately, the practice lives on for home gardeners who can still purchase wound sealants from nurseries, and in countries such as those in Eastern Europe where painting wounds is practiced with religious fervor, despite obvious failures of the paints to prevent decay (Figure 6a-b).
a. b.


Figure 6a-b. Pruning paints did not prevent decay in this horse-chestnut (Aesculus hippocastanum) tree.
Trees and other woody plants have a biochemical response to wounding that requires oxygen. Sealants isolate the tissues from the atmosphere, limiting the oxygen that’s needed for natural wound sealing. Furthermore, artificially sealing wounds can be counterproductive to the main objective of limiting development of wood decay. Wood decay spores in air can fall on pruning cuts immediately after branches are removed (Gauthier et al., 2015). Pruning sealants provide an impenetrable cover and maintain high humidity behind the paint, providing perfect incubation conditions for pathogens. Research indicates that beneficial fungi can also inhabit cut surfaces and that these organisms compete with and inhibit the development of wood decay causing fungi (Schubert et al., 2008). Biological control fungi can be applied after a cut is made to protect the wound from subsequent decay fungus colonization. Tree paints prevent the deposition or application of biological control fungi on cut surfaces.
Research on branch removal shows that leaving the branch collar intact and avoiding flush cuts is more likely to limit decay than the use of pruning paints (Shigo, 1984).
Myth 6: “Trees should be firmly staked at planting”
Nursery-grown shade trees are often rigidly staked to prevent blowdown and damage during cultivation. In some cases, trees are pruned to a long, untapered standard with a bushy top that requires a tight stake to hold it up. Nurseries often remove side branches from the young trunk and while this creates the illusion of a small tree, the practice actually inhibits the development of taper in the trunk (Harris, 1984; Neel and Harris, 1971). Trees without taper will not stand without staking. Poor culture of ornamental trees in nurseries necessitates staking once trees are planted into landscapes because they do not have the structural development in their trunks to stand on their own. Due to these cultivation errors, landscape installers frequently keep the nursery stake and add more stakes to firmly secure the tree in place and further prevent its movement in the landscape.
Staking takes three basic forms: rigid staking, guying, and anchoring. All methods of staking reduce development of taper, increase height growth, and decrease caliper of the developing tree relative to unstaked trees (Figure 7). Moreover, improper staking can result in increased tree breakage either during the staking period or after staking is removed (Figure 8a-b) (Thacker et al., 2018).

Figure 7. Rigid staking prevents development of a strong trunk, causes injury, and slows establishment in the landscape.
a. b.
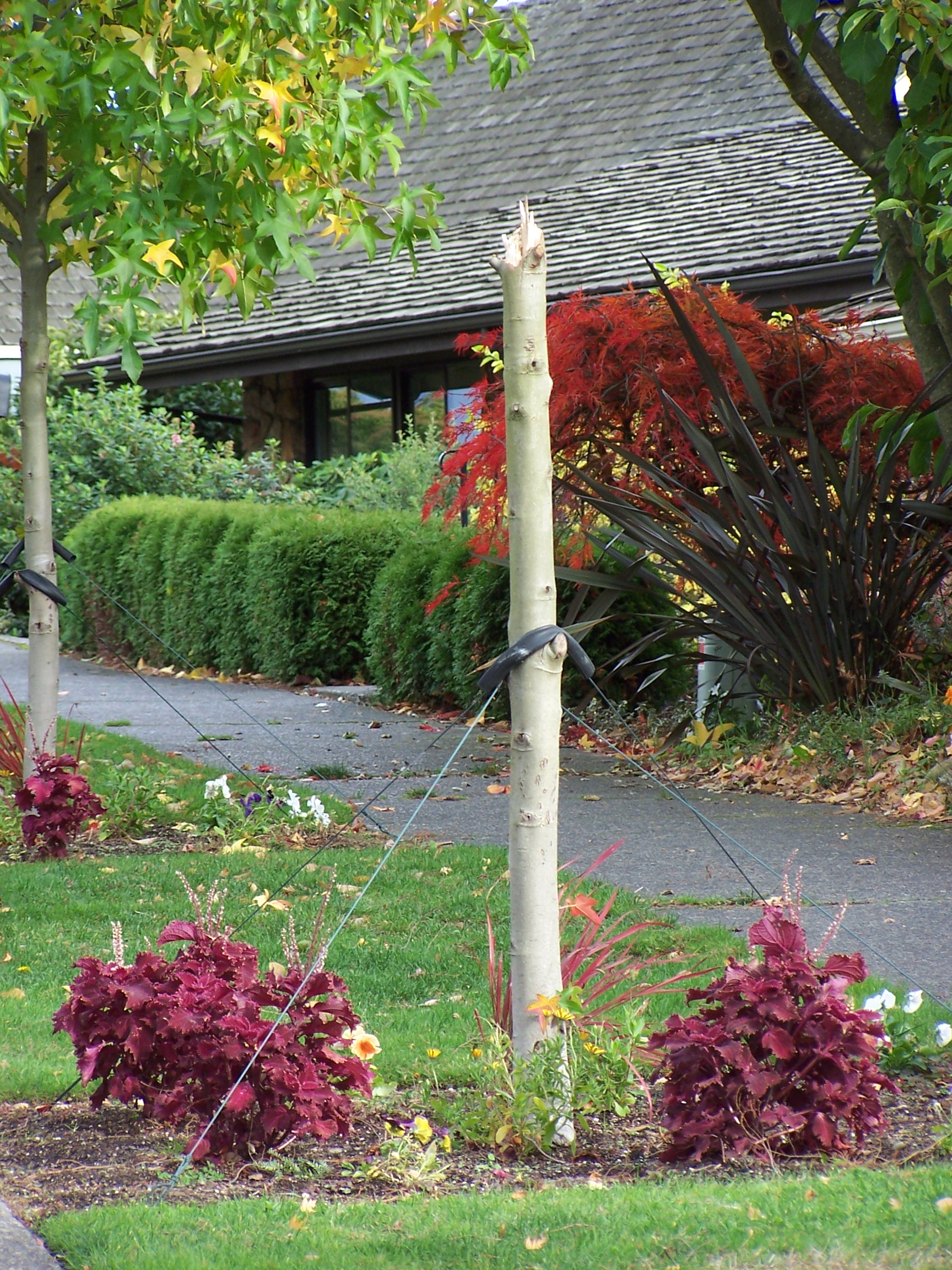
Figure 8a-b. Staking that is too high and tight results in tree breakage above the staking point. When staking has been left on too long, trunks often break after staking is removed.
Decades ago, researchers discovered that movement of the trunk and branches is necessary for the development of trunk taper (Figure 9) (Neel and Harris, 1971). Trees grown in a growth chamber without movement did not develop taper and instead grew taller, while trees in an identical chamber that were hand shaken each day developed significant taper and remained shorter.
Figure 9. Taper is the increase in diameter of the stem near the soil line
Until trees are established in landscapes they may require some staking. In areas of high wind, guying (which involves cables staked to the ground) gives the greatest protection against main stem breakage or blowover (Alvey et al., 2009). Whatever system is used, any such hardware should be removed as soon as the tree can stand on its own:
- The traditional two stakes and ties system is the least harmful to trees staked in landscapes (Figure 10).
- Staking should be low and loose to allow trunk taper to develop naturally.
- Remove all staking material as soon as possible.
- If a tree is not established after a year of staking, it is unlikely to ever establish.

Figure 10. An appropriate staking method. Stakes and ties should be removed as soon as the tree can stand without support or be damaged from wind.
Myth #7: “Wood chip mulches will decrease soil nitrogen and spread pathogens”
With chronic drought and/or record-breaking summer temperatures making it increasingly important to conserve water, many gardeners and groundkeepers are using landscape mulches. The ideal landscape mulch not only moderates soil temperature and conserves water, but also:
- reduces compaction;
- provides nutrients;
- enhances plant growth;
- provides habitat for beneficial insects;
- helps control weeds, pests and disease; and
- reduces the need for pesticides and fertilizers.
In addition, landscape mulches should be readily available, affordable, and easy to apply and replace. A review of the literature on landscape mulches (Chalker-Scott, 2007) determined that organic mulches are overall the best choice, with deep layers of coarse woody material providing most or all of the above-listed benefits (Figure 11). Arborist wood chips (created from leaves and branches chipped up by tree service companies) are a particularly good option as they are generally inexpensive and easy to obtain anywhere trees are managed.
Figure 11. Wood chip mulches are beneficial for trees and shrubs growing under them.
Unfortunately, many people have misconceptions about what arborist wood chips will and will not do. These misconceptions include:
- absorbing nitrogen from the soil;
- suffocating roots by depriving them of oxygen;
- transmitting pathogens to established landscape plants;
- lowering soil pH;
- killing plants through allelopathy; and
- harboring termites, rodents and other pests.
Fortunately, none of these concerns are validated by research. Here are some brief explanations (Chalker-Scott, 2007) targeted to our audience:
- Wood chips will not draw nitrogen from the soil unless they are incorporated into it. When used as mulch, arborist chips have no effect on underlying soil nitrogen levels, except to increase them over time.
- Wood chip mulches, even those made from diseased trees, will not transmit pathogens to healthy plant roots. If diseased chips are incorporated into the soil they could infect plant roots, but field evidence of this is rare. Arborist chips that are stockpiled even for a few days undergo severe pathogen reduction through microbial attack within the pile (Downer et al., 2008).
- Wood chips, or any other organic mulch, will not change the pH of the soil. The soil volume is vast, and any acidification would occur only at the mulch-soil interface where it would quickly be neutralized.
- Wood chips, even those made from black walnut or cedar, will not kill landscape plants. There is no reliable evidence that chemical inhibition from decaying wood actually occurs in a landscape situation.
- Wood chip mulches do not lend themselves to tunnel building like landscape fabric and other sheet mulches do: they collapse. Termites do not eat wood chips unless they have no choice; they are negatively affected by some of the chemicals wood contains. In fact, arborist chip mulches house a number of beneficial insects and other species that naturally control pests.
For arborist wood chip mulches to be the most effective (Chalker-Scott, 2007), they should be:
- coarse – no less than ½” diameter – so water and air can move freely through them;
- applied as soon as possible after chipping both to maximize the materials available to microbes and to capture the nutrients released by their activity in the soil (Figure 12); and
- maintained at a depth of at least 4” to prevent weed growth.
Figure 12. Fresh arborist chips are the best wood-based mulch.
What to do instead: Action items for gardeners and landscape professionals
- Choose appropriate native or introduced tree species based on their suitability to site conditions and ecological function (Chalker-Scott, 2018).
- Prune only broken, dead, or diseased branches in newly transplanted trees.
- Remove branches so that branch collars are left intact – not flush with the trunk.
- Allow cut surfaces to seal naturally: do not use sealing paints or other products.
- Leave lower branches intact to help develop taper and to protect the young bark tissues.
- Avoid mycorrhizal products, but instead use arborist wood chips as a natural inoculant and microbial food source (Chalker-Scott, 2017).
- Remove stakes as soon as possible to avoid injury to the trunk.
- Retain a thick woody mulch layer to reduce weeds and nourish both trees and soils (Chalker-Scott, 2015c).
Horticultural lore is full of apparent “common sense” practices that are not supported with scientific evidence. Gardeners and educators alike must question these practices, asking for supporting evidence from current, relevant, and science-based resources. Developing scientific literacy skills should be part of any professional or volunteer-based training, and can be facilitated through peer-reviewed materials readily available on the web (Chalker-Scott and Daniels, 2016).
Literature Cited
Alvey, A.A., Wiseman, P.E., and B. Kane, B. (2009). Efficacy of conventional tree stabilization systems and their effect on short-term tree development. Journal of Arboriculture, 35(3):157-164. http://auf.isa-arbor.com/request.asp?JournalID=1&ArticleID=3104&Type=2
Appleton, B., Koci, J. French, S., Lestyan, M., and Harris, R. (2003). Mycorrhizal fungal inoculation of established street trees. Journal of Arboriculture, 29:107-110. http://joa.isa-arbor.com/request.asp?JournalID=1&ArticleID=83&Type=2
Bayala, J., Teklehaimanot, Z., and Ouedraogo, S.L. (2004). Fine root distribution of pruned trees and associated crops in a parkland in Burkina Faso. Agroforestry Systems, 60:13-26.
Carpio, L.A., Davies, F.T., and Arnold M.A. (2005). Arbuscular mycorrhizal fungi, organic and inorganic controlled-release fertilizers: effect on growth and leachate of container-grown bush morning glory (Ipomoea carea ssp. fistulosa) under high production temperatures. Journal of the American Society for Horticultural Science, 130: 131-139.
Chalker-Scott, L. (2018). Are native tree and shrubs better choices for wildlife in home landscapes? WSU Extension Fact Sheet FS300E. http://cru.cahe.wsu.edu/CEPublications/FS300E/FS300E.pdf
Chalker-Scott, L. (2017). A gardener’s primer to mycorrhizae: understanding how they work and learning how to protect them. WSU Extension Fact Sheet FS269E. http://cru.cahe.wsu.edu/CEPublications/FS269E/FS269E.pdf
Chalker-Scott, L. (2015a). How Plants Work: The Science Behind the Amazing Things Plants Do. Timber Press, Portland, OR.
Chalker-Scott, L. (2015b). Nonnative, noninvasive woody species can enhance urban landscape biodiversity. Arboriculture and Urban Forestry, 41(4):173-186. https://www.researchgate.net/publication/281751812_Nonnative_noninvasive_woody_species_can_enhance_urban_landscape_biodiversity
Chalker-Scott, L. (2015c). Using arborist wood chips as a landscape mulch. WSU Extension Fact Sheet FS160E. http://cru.cahe.wsu.edu/CEPublications/FS160E/FS160E.pdf
Chalker-Scott, L. (2007). Impact of mulches on landscape plants and the environment – a review. Journal of Environmental Horticulture, 25(4):239-249. http://hrijournal.org/doi/pdf/10.24266/0738-2898-25.4.239
Chalker-Scott, L. and Daniels, C.H. (2016). Scientific Literacy for the Citizen Scientist. WSU Extension Manual EM100E. http://cru.cahe.wsu.edu/CEPublications/EM100E/EM100.pdf
Corkidi, L., Allen, E.B., Merhaut, D., Allen, M.F., Downer, J. Bohn, J., and Evans, M. (2004). Assessing the infectivity of commercial mycorrhizal inoculants in plant nursery conditions. Journal of Environmental Horticulture, 22:149-154. http://hrijournal.org/doi/pdf/10.24266/0738-2898-22.3.149
Corkidi, L., Allen, E.B., Merhaut, D., Allen, M.F., Downer, J. Bohn, J., and Evans, M. (2005). Effectiveness of four commercial mycorrhizal inoculants on the growth of Liquidambar styraciflua in plant nursery conditions. Journal of Environmental Horticulture, 23:72-76. http://hrijournal.org/doi/pdf/10.24266/0738-2898-23.2.72
Downer, A.J., Crohn, D., Faber, B., Daugovish, O., Becker, J.O., Menge, J.A., and Mochizuki, M.J. (2008). Survival of plant pathogens in static piles of ground green waste. Phytopathology, 98:574-55. https://apsjournals.apsnet.org/doi/pdf/10.1094/PHYTO-98-5-0547
Gauthier, N.W., Fountain, W.E., and Missun, T. (2015). Tree wounds invitations to wood decay. University of Kentucky Plant Pathology Fact Sheet PPFS-OR-W-01. https://plantpathology.ca.uky.edu/files/ppfs-or-w-01.pdf.
Harris, R.W. (1984). Effects of pruning and staking on landscape trees. Journal of Environmental Horticulture 2:140-142. http://hrijournal.org/doi/pdf/10.24266/0738-2898-2.4.140
Jones, M., Sinclair, F.L., and Grime, V.L. (1998). Effect of tree species and crown pruning on root length and soil water content in semi-arid agroforestry. Plant and Soil, 201:197-207. https://link.springer.com/content/pdf/10.1023%2FA%3A1004324616942.pdf
Linderman, R.G. (1988). Mycorrhizal interactions with the rhizosphere microflora: the mycorrhizosphere effect. Phytopathology, 78:366-371. https://www.apsnet.org/publications/phytopathology/backissues/Documents/1988Articles/Phyto78n03_366.PDF.
Martin, C.A., Whitcomb, S.A. , and Stutz J.C. (2010). Effects of frequent shearing on root growth and mycorrhizal colonization of two landscape shrubs. HortScience, 45(10):1573-1576. http://hortsci.ashspublications.org/content/45/10/1573.full
Neel, P.L. and Harris, R.W. (1971). Motion-induced inhibition of elongation and induction of dormancy in Liquidambar. Science, 173:58-59.
Reichard, S.H. and White, P. (2001). Horticulture as a pathway of invasive plant introductions in the United States. BioScience, 51(2): 103-113. https://academic.oup.com/bioscience/article/51/2/103/390610.
Schubert, M., Fink, S., and Schwarze F.W.M.R. (2008). Evaluation of Trichoderma spp. as a biocontrol agent against wood decay fungi in urban trees. Biological Control, 45:111-123. https://ac.els-cdn.com/S1049964408000029/1-s2.0-S1049964408000029-main.pdf?_tid=f129ff22-5edb-4730-a883-0f58d38bcaf2&acdnat=1541012443_ae85aef42d5b91d419abf689bfcf6721
Shigo, A.L. (1984). Tree decay and pruning. Arboricultural Journal, 8(1):1-12. DOI: 10.1080/03071375.1984.9746646
Shigo, A.L. and Shortle, W. (1983). Wound dressings: results of studies over 13 years. Journal of Arboriculture, 12:317-329. http://joa.isa-arbor.com/request.asp?JournalID=1&ArticleID=1923&Type=2
Thacker, Martin, H.J. and Slater, D. (2018). Supporting failure? Damage inflicted to establishing trees in London by a range of tree support and protection systems. Arboricultural Journal, DOI: 10.1080/03071375.2018.1493874.






