Journal of the NACAA
ISSN 2158-9429
Volume 12, Issue 1 - June, 2019
Case Study Evaluating Collection Technique and Sample Handling on Nematode Analysis
- Burdine, B. , Regional Agronomy Specialist, Mississippi State University Extension Service
ABSTRACT
Soybean yield losses by nematodes has been rising for over 10 years in NE Mississippi but foliar injury is often attributed to diseases. Nematode samples submitted to a laboratory often show low nematode populations despite field symptomology indicating a more severe infestation. Growers routinely receive below-threshold analysis yet still have yield losses. A case study was conducted to determine if collection technique and sample handling negatively affected sample analysis. When grower and Extension professional sampled the same fields, the Extension professional tended to find higher populations of root-knot nematodes. Soybean cyst populations were found at random. Following university sampling recommendations did not result in higher nematode counts for root-knot or cyst nematodes. No population trend was seen from holding samples for 0, 30 or 78 hours prior to delivery to laboratory. Spatial variability and egg hatch are considered primary reasons for analysis variances. Nematodes are extremely difficult to sample accurately which can result in a false-negative analysis. Growers that see foliar injury but receive a below-threshold analysis should consider submitting a follow-up sample to verify results and eliminate nematodes before assuming a disease is the primary causal agent.
INTRODUCTION
Plant parasitic nematodes are a hidden threat to almost every crop grown for food and fiber. Nematodes often go unnoticed because the tiny worm-like invertebrates usually require a microscope for viewing. Being unnoticed leads to poor management.
The top three species affecting Mississippi soybean fields are: soybean cyst (Heterodera glycines) (SCN), southern root-knot (Meloidogyne incognita) (RKN) and reniform (Rotylenchulus reniformis) nematodes. All three species are found throughout Mississippi however SCN populations seem to be more prevelant in northeast Mississippi. It is possible to have yield losses of 30% from SCN even when foliar symptoms are unnoticeable (Allen et al., 2017; Noel 1992; Noel and Edwards, 1996; Wang et al., 2003). When symptoms are noticed, damage is often misdiagnosed as being caused by another pathogen (Mueller et al., 2016) or nutrient deficiency. This is understandable as symptoms can be very similar; all three issues can cause wilting, reduction in nitrogen-fixation, stunting and yellowing of foliage (Figures 1 and 2).
Figure 1. Mortality and chlorosis from soybean cyst nematode in Pontotoc County, MS.

Figure 2. Severe bronzing and necrosis from root-knot nematode in Alcorn County, MS.
Stunting, yellowing and wilting are caused when parasites feed so heavily on plant roots that water and nutrient demands can no longer be supported. Root galls may occur when attacked by RKN females which cause the tissue calluses. Rhizobium nodule formation may be reduced by nematode presence. It is believed that nematodes prevent rhizobia bacteria from attaching properly to roots, which reduces nitrogen available to a plant. Soybean producers rely on this “free” nitrogen to supply most nitrogen requirements for the crop. A reduction in nodules will lower soybean yield or require increased fertilizer expenses.
Allen et al. (2017) conducted a multi-state survey to estimate yield loss by pathogen and stated that between 2010 and 2014, SCN caused at least double the yield loss per year than any other soybean nematode or disease. SCN losses ranked first or second in each year in the southern U.S.
Infestation of SCN in Mississippi was first reported in 1957, however soybean yields rebounded in the late 1970’s when a naturally-occurring fungal parasite reduced SCN populations (Allen et al., 2017; Moore et al., 1991). Unfortunately, SCN damage has been on the rise in northeast Mississippi since the early 2010s. This is likely due to SCN developing resistance to the fungal parasite (Burdine, personal experience). Rotation is the primary control method however this is not always effective. SCN eggs have been found to remain viable in the soil without a suitable host for three, (Moore et al., 2012) seven (Wight et al., 2011) and twelve years (Alston and Schmitt, 1987).
Rationale for Case Study
Mississippi State University Extension recommendations for nematode analysis include: sample after harvest; collect a minimum of 20 cores from 6 to 8 inch depth; collect cores beneath previous crop row; mix thoroughly; submit 1 pint soil/sample; and keep samples cool until delivered to laboratory (Allen, 2012). For convenience, samples are routinely collected over several days and stored in a hot vehicle before being delivered for analysis (Burdine, personal experience). Failure to keep samples cool may result in an inaccurate analysis. Based on field observations, growers are not consistent in depth of sampling or sampling beneath old crop rows. An inaccurate analysis falsely indicating a below-threshold populations may prompt producers to search for other culprits. This could lead to expensive attempts to manage a disease that does not exist or to apply fertilizer when soils are nutrient sufficient.
The objectives of this case study were to determine whether improper collection technique or sample handling altered laboratory results. For collection technique, samples were collected by growers without training and by a trained Extension professional. This comparision was to determine if a properly trained Extension Specialist would find higher populations of nematodes. For sample handling, it was expected that failure to follow recommended handling procedures would result in heat-related mortality. We hypothesized that samples subjected to 30 and 78 hour heat stress would result in lower nematode population counts.
METHODS
The case study was conducted using five producers from separate counties in northeast Mississippi. Samples were collected on Tuesday, August 22 and Tuesday, August 29, 2017. Statistical analysis was not performed as this was a case study.
Producers were recommended by the local Extension Agent from each participating county. Each producer was asked if they had “yellow or stunted spots in soybean fields”. If yes, the field was located and the Extension Specialist (ES) marked a 1-acre containment area. Growers (G) were asked to collect a nematode sample within the containment area. Growers were not provided any sampling instructions or guidance other than a nematode sample was needed. Each grower was provided a clean sample probe and plastic bucket. After collection, the sample was labeled as G. After the grower sample was completed, the ES entered the containment area and collected a sample consisting of 30 cores from a depth of six inches. The sample was thoroughly mixed and subdivided into three sub-samples identified as ES-0, ES-30 and ES-78. The ES-0 sample was placed inside an ice cooler to protect it from heat and delivered to the nematode laboratory within four hours. ES-30 and ES-78 samples were stored inside the vehicle and not protected from sunlight or heat. ES-30 samples remained in the vehicle for 30 hours until shipment via U.S. Postal Service (USPS) at 5pm Wednesday. The ES-78 and G samples remained in vehicle for 78 hours until shipment via USPS at 5pm Friday. After-hours drop-off to USPS guaranteed samples would need an additional day for delivery at laboratory. All samples were analyzed at the Mississippi State University Extension Plant Pathology Laboratory.
To compare collection techniques, nematode population counts from the ES-78 and G samples were performed. Both samples were held for 78 hours inside a hot vehicle which reached a maximum internal temperature during storage of 118◦F.
To compare sample handling, nematode population counts from the ES-0, ES-30 and ES-78 samples were performed. The maximum internal temperature during storage reached 118◦F.
RESULTS
Laboratory analysis indicated below-threshold populations for reniform, lesion, spiral, lance, stunt and sheath nematode species in all samples and will not be discussed. RKN populations exceeded economic injury threshold in four of five fields. SCN populations exceeded economic injury threshold in five of five fields. The SCN populations are reported as the total of juveniles, hatched juveniles (hatched in laboratory), and cyst stage (encysted females).
Collection Technique - RKN populations were highest in ES-collected samples in three of five fields (Figure 3). Each of these three locations were above the economic injury threshold of 500 nematodes/pint soil. The G-collected samples were higher in the remaining two fields however populations were extremely low and below thresholds at 16 and 24 RKN respectively. In fields with considerable RKN populations, the ES identified 3 of 3 above-threshold fields while growers identified 1 of 3.
Every SCN sample was above the economic injury threshold of 1 nematode/pint of soil (Figure 4). ES samples were higher in 2 of 5 fields and the populations were extremely high. Grower samples were higher in 3 of 5 fields. Although ES and G samples show different populations, each sample reaffirms that nematode populations are elevated in northeast Mississippi soybean fields.
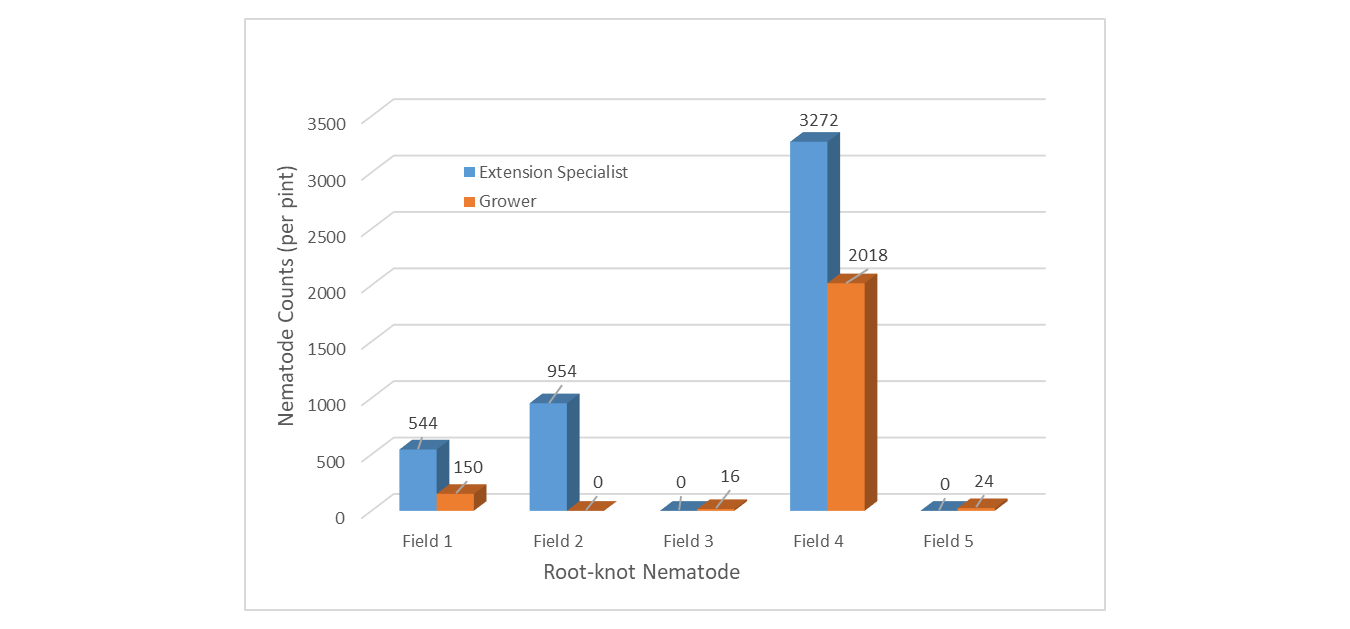
Figure 3. Root-knot nematode populations sampled by Extension Specialist compared to growers.
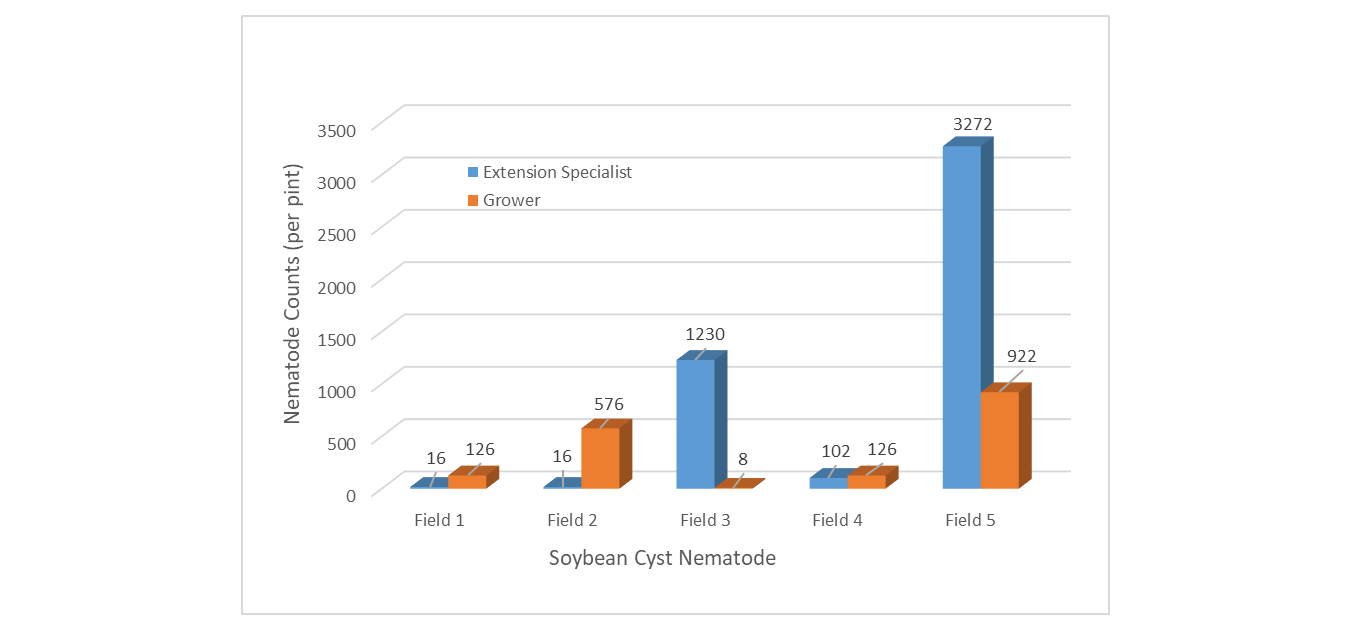
Figure 4. Soybean cyst nematode populations sampled by Extension Specialist compared to growers.
Sample Handling - RKN population counts were only slightly affected by increased hours held in a hot vehicle in 3 of 5 fields (Figure 5). Most fields did not show the expected pattern of decreasing counts with increasing hours in hot vehicle. Field 3 was the only site to show the anticipated mortality of nematodes from being held in a hot vehicle.
For SCN, holding samples for 78 hours saw highest populations in 3 of 5 fields (Fields 1, 3, and 5) (Figure 6). Conversely, the 78-hour hold provided the lowest populations in the two remaining fields (Fields 2 and 4). SCN populations did not show decreasing counts with increasing hours in a hot vehicle as was expected.
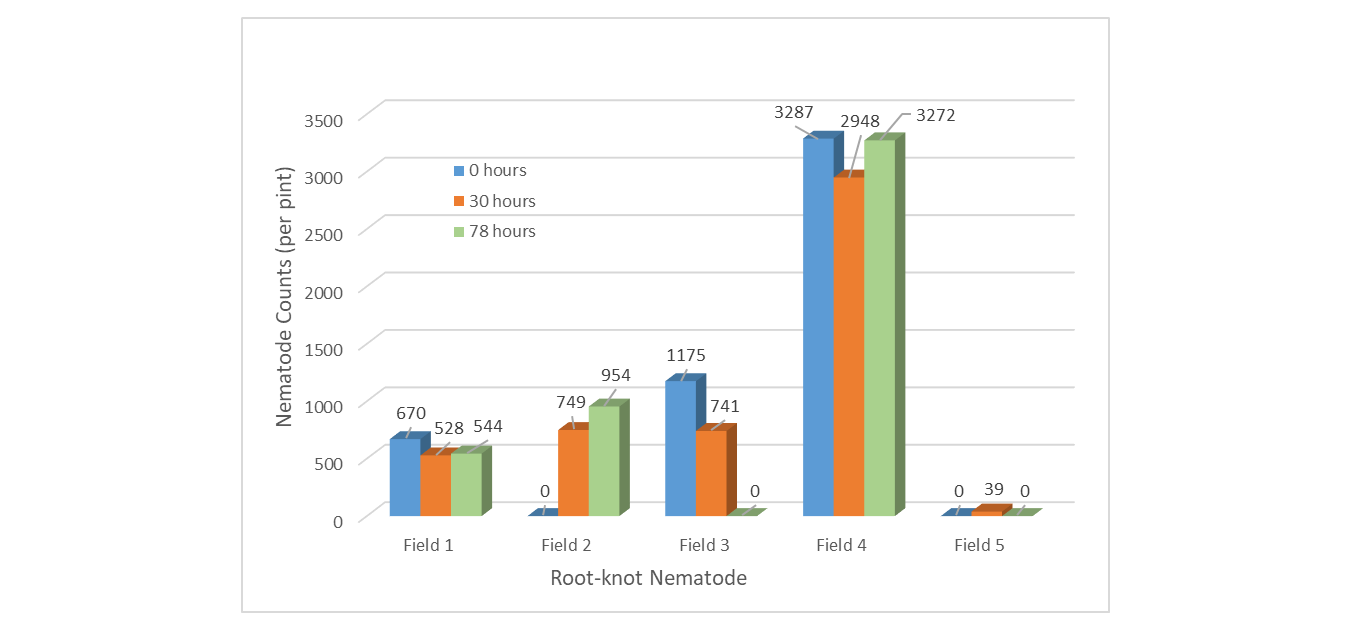
Figure 5. Root-knot nematode populations when stored in hot vehicle for 0, 30 and 78-hours.
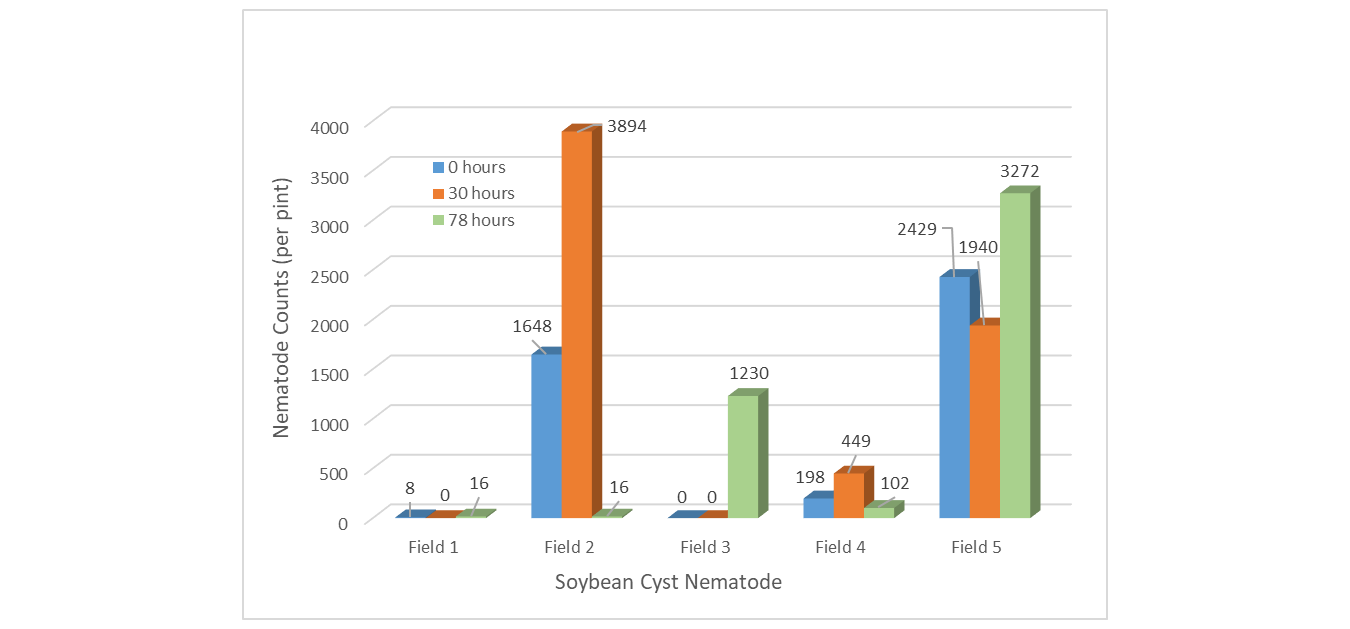
Figure 6. Soybean cyst nematode populations when stored in hot vehicle for 0, 30 and 78-hours.
DISCUSSION
Nematode sampling analysis did not follow expected patterns for either study objective. For collection technique, the ES tended to locate higher RKN populations than did the Grower. Two fields showed a false-negative from grower sampling. Highest SCN populations tended to be found at random and experience in sampling methodology did not translate into greater accuracy by the ES.
For sample handling, extreme care of samples and immediate delivery to the laboratory (0-hr) provided greater populations in three fields for RKN and zero fields for SCN. The treatment with the least care for sample integrity (78-hr) provided the highest populations of RKN in one field and highest SCN populations in two fields. The intermediate treatment (30-hr) had highest populations in one RKN and two SCN fields. The higher populations from samples stored for 30 or 78 hours in a hot vehicle contradicts traditional recommendations for proper sample handling. The question now becomes whether extreme care of samples is necessary or does spatial variance of populations mask any handling effects.
Nematode populations tend to cluster spatially within a field (Noe and Campbell, 1985) due to temperature, soil type, moisture, and host plant. Hatching of SCN eggs is known to be controlled by temperature, host, and time (Yen et al., 1995). Within one egg mass, some eggs hatch at a certain number of hours, other eggs hatch at specific temperature, and still other eggs hatch when root exudates are present. The wide range of egg hatch criteria could explain why some of the 30-hr and 78-hr samples had higher counts.
Environmental conditions prior to sampling combined with adult mortality and successive hatchings tend to make nematode analysis inaccurate (Yen et al., 1995; D'Addabbo et al., 2004). This case study demonstrates that following proper procedures for nematode sampling can still result in a false negative analysis. If laboratory results are negative, yet a field shows symptomology such as stunting or yellowing, it is justifiable to submit another sample to overcome possible spatial variation and hatching effects. This case study further demonstrates the increasing geographical distribution of above-threshold levels of RN and SCN nematodes across northeast Mississippi.
LITERATURE CITED
Allen, T. 2012. Soybean cyst nematode. Coop. Ext. Serv. Publ. 1293. Mississippi State Univ., Mississippi State, MS.
Allen, T.W., Bradley, C, Byamukama, E. and Chilvers, M. 2017. Soybean yield loss estimates due to diseases in the United States and Ontario, Canada, from 2010 to 2014. Plant Health Prog. 18:19-27.
Alston, D. G., and Schmitt, D. P. 1987. Development of Heterodera glycines life stages as influenced by temperature. J. Nematol. 20:366-372.
D'Addabbo, T., Sasanelli, N, Greco, N, Stea, V. and Brandonisio, A. 2004. Effect of water, soil temperatures, and exposure times on the survival of the sugar beet cyst nematode, Heterodera Schachtii. Phytopathology 95:339-344.
Moore, W. F., Fox, J. A., and Patel, M. V. 1991. Soybean cyst nematode. Coop. Ext. Serv. Publ. 1293. Mississippi State Univ., Mississippi State, MS.
Mueller, D. S., Wise, K. A., Sisson, A. J., Smith, D. L., Sikora, E. J., Robertson, A. E., and Bradley, C. A. 2016. A farmer’s guide to soybean diseases. American Phytopathological Society, St. Paul, MN.
Noe, J. P., and Campbell, C. L. 1985. Spatial pattern analysis of plant-parasitic nematodes. J. Nematol 17:86-93.
Noel, G. R. 1992. History, distribution, and economics. Pages 1-3 in: Biology and Management of the Soybean Cyst Nematode. R. D. Riggs and J.A. Wrather, eds. American Phytopathological Society, St. Paul, MN.
Noel, G. R., and Edwards, D. I. 1996. Population development of Heterodera glycines and soybean yield in soybean-maize rotations following introduction into a noninfested field. J. Nematol. 28:335-243.
Wang, J., Niblack, T. L., Tremain, J. A., Wiebold, W. J., Tylka, G. L., Marett, C. C., Noel, G. R., Myers, O., and Schmidt, M. E. 2003. Soybean cyst nematode reduces soybean yield without causing obvious aboveground symptoms. Plant Dis. 87:623-628.
Wight, J. P., Allen, F. L., Donald, P. A., Tyler, D. D., and Saxton, A. M. 2011. Impact of crop rotation and bio-covers on soybean cyst nematode. Online. Plant Health Prog. 10.1094/PHP-2010-0930-04-RS.
Yen, J. H., Niblack, T. L., and Wiebold, W. J. 1995. Dormancy of Heterodera glycines in Missouri. J. Nematol. 27:153-163.

