Journal of the NACAA
ISSN 2158-9429
Volume 12, Issue 1 - June, 2019
Evaluation of Low-Cost, Non-Invasive Hive Monitoring Tools for Overwintering Honeybee Hives
- Jones, K. M., County Director, Master Beekeeper candidate, Colorado State University Extension
Kephart, R., Master Beekeeper candidate, University of Montana student
Slocum, D.L., Master Beekeeper candidate, University of Montana student
Storm, A., Master Beekeeper candidate, University of Montana student
Thomas, A., Master Beekeeper candidate, University of Montana student
Wicker, R., Master Beekeeper candidate, University of Montana student
ABSTRACT
Overwinter colony loss is a significant concern for large and small beekeeping operations. To care for colonies with a prolonged winter confinement period, beekeepers must learn how to recognize and promptly deal with colony health and queen problems during the summer and fall, with an eye to ensuring that colonies enter winter clustering in the best possible condition. Evaluating how these management decisions have affected overwintering bees is difficult in extreme weather without risking bees’ health. Low cost tools such as digital meat thermometers, infrared thermometers, humidity gauges, acoustic meters and lower cost thermal cameras were tested in six apiary locations across the northern United States and Canada to determine their efficacy in monitoring overwintering hive health. Of the tools tested, the digital thermometers were the most effective across the six states, though the acoustic meter and digital meat thermometers were also accurate under specific environmental and hive conditions. The humidity meters and low-cost thermal imaging cameras were not effective in extreme weather conditions.
Introduction
Beekeepers in the United states lost an estimated 21% of their hives over the winter of 2016-2017 (BeeInformed Project, 2017). Until recently, there was little a beekeeper could do to check on the health of a hive during the winter other than to observe the hives visually on a warm day, look for dead bees in the snow outside of the hive, or attempt to listen for buzzing inside the hive. Modern technological developments provide beekeepers new opportunities to measure and monitor beehive health through the winter months. Accurate information on the health of a hive during the winter is valuable to beekeepers as it will inform future practice and potentially allow them to intervene with a failing hive prior to the loss of the colony (Graham, 2015). The development of thermal imaging cameras has given beekeepers the ability to conduct non-intrusive winter hive checks and collect important data to improve their decision making (Bromenshenk, 2016), but the price of the camera may be a barrier to widespread use among small beekeeping operations. Other, lower cost technologies also have the potential to provide useful insights into the health of the colony.
Honey bees survive the winter cold through a process of active thermoregulation where they form a cluster and generate heat by vibrating their thoracic flight muscles (Jones and Oldroyd, 2006; Southwick and Heldmaier, 1987; Harris, 2009). The success of this process is dependent, at least in part, upon adequate honey supplies to fuel their physical heat production, adequate colony size to be able to have a large enough cluster to effectively heat themselves, and adequate ventilation to remove humidity and carbon dioxide generated through respiration. An accurate understanding of this process and the waste products it produces (heat, humidity and noise) may allow beekeepers the ability to gain some insight into the health of the colony (Meikle and Holst, 2015) through a nonintrusive winter hive check with low-cost equipment.
Clusters commence to form when the interior temperature of the hive falls to 18°C (Southwick and Heldmaier, 1987). Bees maintain cluster temperature by vibrating their wing muscles, which produces both heat and noise (Meikle and Holst, 2015). On average, colonies maintain a core cluster temperature of 21.3° C (Storch, 1985), and colonies that are unable to maintain a core cluster temperature of at least 15° C are at risk (Fahrenholz et al., 1989).
Clusters lose heat to the surrounding air and this heat, along with the humidity generated through respiration, rises within the hive (Hesbach, 2016). While the total interior of the physical hive is not maintained at the same temperature as the bee cluster (Heinrich, 1993), the rising warm and humid air should cause the top entrance of a hive to be warmer and more humid than the bottom entrance. It may be possible to identify a safe temperature and humidity differential between the bottom entrance temperature and the top hive entrance that will give insight into the amount of heat the colony is producing and, by correlation, the health of the colony.
In addition, the size of the cluster and the position of the cluster within the hive will have an impact on the volume of the noise escaping through the upper hive entrance, but it may be possible to gain insight into the health of the colony by monitoring the volume of the buzz escaping the hive.
Materials and Methods
A total of thirty hives in six different bee yards spread across North America were included in this study. The bee yards were located near the communities of Jonesville MI, Bowie MD, Bow NH, Salida CO, Wilton IA, and Lac La Biche AB, Canada. Twenty-four hives had live bees in them at the beginning of the study and 6 hives, one in each yard, were set up as controls without any bees. Twenty-six of the hives were Langstroth hives and four were top bar hives. The hives in Lac La Biche were wrapped in insulated plastic blankets, the hives in Bow were wrapped with tar paper and the rest of the hives were unwrapped. All five hives in each location were monitored for 21 days between January 20 and February 20, 2019. A variety of low-cost tools were used to monitor the hives including non-contact infrared spot thermometers, digital meat thermometers, humidity gauges and decibel meters. Four of the locations also used thermal cameras to monitor their hives. Ambient temperature and relative humidity were also tracked in each location. Over the course of the research the ambient temperature ranged from -22°C to +10° C, and the ambient relative humidity ranged from 24% to 94.8% across the five locations.
Temperature was monitored at the upper and lower entrances in four separate ways. All the hives were tested by inserting a meat thermometer into both of the hive entrances. Twenty-five of the hives were monitored by pointing an infrared beam into the entrances and locating the warmest temperature. Five hives were monitored by pointing an infrared beam on the outside of the hive body just below the entrances. 20 of the hives also had their temperature monitored with thermal imaging cameras. Humidity and the acoustic volume were measured by holding sound and humidity meters next to the upper entrance and recording the readings, or by inserting a probe into entrances with the sensors attached via zip ties.
At the end of one week of monitoring it was determined that a few of the tools were not practical or sufficiently non-intrusive and were discontinued at some locations. The digital meat thermometer was too intrusive a tool as many bees would come out of the hive when the probe was inserted into the upper entrance regardless of the ambient temperature. As the goal of the study is to identify non-intrusive equipment to complete a winter hive check, the use of the digital meat thermometer was discontinued in several apiaries. In some locations, the humidity sensors and the thermal cameras were not able to provide readings at the low ambient temperatures and they were also discontinued. The decibel readers were used throughout the research, but ambient sound in most locations made it difficult to take accurate readings.
All data were recorded into Microsoft Excel, and all statistics were calculated using IBM SPSS, version 25. Comparison of means of active hives were compared with controls in each apiary location (Gliner et al., 2009). Significance levels were established a priori at p = .05 (Morgan et al., 2011).
Results
Comparing temperature means from the active hives versus control hives showed some interesting results. For those apiaries utilizing Langstroth Hives with upper ventilation, temperature differences were observed (p <.001 for each location). Because many apiaries will not be set up with control hives of their own, we also compared upper temperature readings from infrared thermometers with ambient (outside) temperatures, similar to what many stationary beekeepers might be able to replicate. This strategy also showed to be a successful means to evaluate hive activity (p <.001). See Table 1 for more details.
Table 1: Statistical results for selected temperature comparisons. T-test comparisons were made between infrared thermometer temperatures in upper ventilation active hives and controls (IR Top Vent vs. Control), and between upper ventilation and ambient temperatures for active hives (IR Vent Temp vs. Control). Numerous equipment failures occurred at the Wilton, IA site due to extremely low ambient temperatures.
| t | df | p | |
| IR Top Vent vs. Control | |||
| Salida, CO | 7.383 | 55 | <0.001 |
| Bow, NH | 5.343 | 43 | <0.001 |
| Lac La Biche, AB | 12.197 | 31 | <0.001 |
| Wilton, IA | 0.707 | 45 | 0.483 |
| Bowie, MD | 8.117 | 31 | <0.001 |
| Jonesville, MI | 2.418 | 79 | 0.018 |
| IR Vent Temp vs. Ambient | |||
| Salida, CO | 15.084 | 55 | <0.001 |
| Bow, NH | 4.196 | 39 | <0.001 |
| Lac La Biche, AB | 23.400 | 94 | <0.001 |
| Jonesville, MI | 5.575 | 79 | <0.001 |
For those apiaries that were able to take humidity readings within their hives, this was also shown to be an accurate measure when compared with control hives (t = 5.240, p <.001) and against ambient humidity (t = 11.382, p <.001). Several of our test locations were not able to insert the humidity probe into upper entrances due to mouse excluders or other physical limitations.
One of the strategies that had mixed results was the use of an acoustic meter when compared with control hives. In some apiary locations, the use of an acoustic meter worked well (p <.001 to p = .037) (Figure 1). Hive 1 provided an interesting example where acoustic data revealed the time period when a colony succumbed to winter. In other situations, ambient noise such as wind, traffic, and other noises interfered with the accuracy of this instrument in this study (p = .558; p = .656).
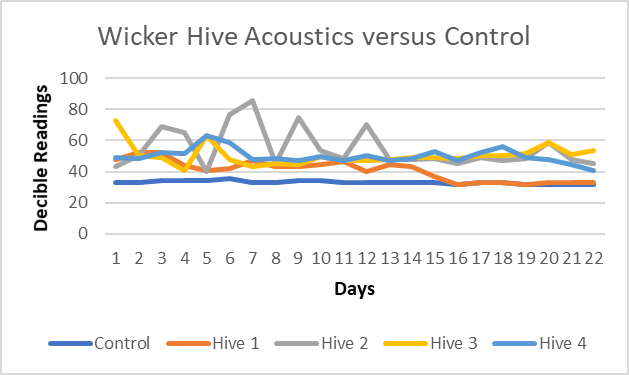
Figure 1. Acoustic decible levels for 4 active hives and 1 control at the "Wicker" apiary at Lac La Biche, AB, Canada.
In some apiaries, different hive equipment was utilized such as a top bar hive. These hives do not have a ventilation hole for taking temperature measurements, so lower entrances were utilized for temperature readings. In this location, the use of the lower entrances was an accurate measurement (t = 7.677, p <.001).
Of note, one “active” hive in an apiary collected a mouse during the study. Mice will often seek shelter in overwintering hives, typically to the mouse's detriment. The mouse likely had moved into the hive prior to our study, but the presence of the mouse may have skewed the results of the temperature readings. Initial statistics on the temperature differences versus controls showed a statistical difference (t = 10.047, df = 95, p<.001). Upon learning of the mouse, we pulled that hive from the dataset and still found the differences were statistically significant (t = 13.862, df = 71, p <.001).
Table 2 provides a summary of the various tools and their relative effectiveness.
Table 2. Relative effectiveness of low-cost monitoring tools. Effectiveness was ranked as poor, fair, good or excellent.
| Tool | Effectiveness | Comments |
| Meat thermometer | Good | Inserting probe into cluster causes bees to become aggressive; slower method to measure temperatures. |
| Infrared thermometer -- surface | Good | Reading taken on hive surfaces showed differences on front of hives, but not on back of hives. Difficult on insulated hives. Instant results |
| Infrared thermometer -- Vents | Excellent | Temperature differences from upper vents compared with lower entrances were reliable. Able to take readings on insulated hives; instant results. |
| Acoustic meters | Good/Excellent | Apiary locations in quiet areas found good success with acoustic readings; sensitive to wind and other ambient noise from suroundings. |
| Humidity probes | Poor/Fair | Differences were shown in some apiaries, others did not see significant differences or were unable to use due to size of probe. |
| Low-cost infrared cameras | Poor/Fair | Most expensive tool utilized in this study, some models only operate at temperatures above freezing; professional IR camera was accurate. |
Discussion
The goal of this research was to determine if beekeepers are able to gather useful data on the health of their hives with low cost, nonintrusive equipment. We have determined that it is possible and that the data collected is both valid and useful. We have not identified any specific temperature, relative humidity or acoustic ranges, differentials or benchmarks as each beekeeper is dealing with a different context, but we do suggest that local beekeepers will be able to determine their own benchmarks that are applicable in their own context. This will require beekeepers to determine which data set will be most valuable to them, e.g. urban beekeepers may not benefit from the data collected with a low-cost decibel reader due to ambient noise. Beekeepers will need to prepare their hives for the equipment they plan to use, such as ensuring that the entrances are large enough for access by the tools they will use. Most importantly, they will need to track their own data over time to determine the benchmarks that apply to their own apiary.
This research validates the use of low-cost tools for data collection, and enabled identification of a few basic parameters for the selection of these tools. In general, we have determined that any tools that are inserted into the upper entrance are invasive from the bee's perspective and will draw the bees out of the hive. Thermal imaging cameras provide the most accurate data for uninsulated hives, but most cameras that are able to function at extreme low temperatures are expensive and do not meet the criteria of low cost. Infrared thermometers, decibel readers and humidity sensors are all relatively low cost and provide good data, but beekeepers need to ensure the tools are rated for the normal low temperatures they experience in their location.
This research indicates that new low-cost technology can be very useful for beekeepers, but we are convinced that the data they provide is only useful when it is interpreted by an informed and observant beekeeper. Beekeepers who understand their hives and are willing to track data over a long period of time should be able to combine the data with their week-to-week regular observations and develop their own apiary benchmarks and protocols that will help to guide their decision making as they work to help their hives survive the winter.
Consideration for future study
Significant research has been conducted into wintering bees (e.g. Shaw, et al., 2010), but this study raises questions for further research that would benefit hobby beekeepers. Tracking the status of cluster temperature within the winter hive is historically difficult and generally highly invasive. Our research demonstrated some non-invasive protocols and inexpensive tools were successful in tracking the winter cluster temperatures. Continuing this research from the winter season into the spring season until all hives have either survived or succumbed to winter may provide new insights into the value of the data and may provide a clearer understanding of acceptable temperature differentials. Secondly, it may be valuable to track the weight of the hive along with the rest of the data as this may help the beekeeper narrow down why the bees are thriving or failing. A third line of research that this study suggests centers around what beekeepers can do if they discover that one of their hives is failing when the ambient temperature is too low to open the hive.
References Cited
BeeInformed Project. (2017). Honey bee colony losses 2016-2017: Preliminary results, August 28, 2017. Available at https://beeinformed.org/
Bromenshenk, J.J. (2016). Should an infrared camera be in your toolkit? Winter uses for thermal cameras. Bee Culture, available at: https://www.beeculture.com/should-an-infrared-camera-be-in-your-toolkit/
Fahrenholz, L., Lamprecht, I., and Schricker, B. (1989). Thermal investigations of a honey bee colony: thermoregulation of the hive during summer and winter and heat production of members of different bee castes. Journal of Comparative Physiology 159 (5):551-560.
Gliner, J. A., Morgan, G. A., and Leech, N. L. (2009). Research methods in applied settings: An integrated approach to design and analysis, 2nd edition. New York: Routledge Taylor and Francis Group.
Graham, J. M. (2015). The Hive and the honey bee: A new book on beekeeping which continues the tradition of Langstroth on the hive and the honeybee. Hamilton, IL: Dadant & Sons.
Harris, J.L. (2009). Development of honey bee colonies on the Northern Great Plains of North America during confinement to winter quarters. Journal of Apicultural Research 48(2):85-90.
Heinrich, B. (1993). Hot-blooded insects: Strategies and mechanisms of thermoregulation. Berlin: Springer-Verlag Berlin Heidelberg.
Hesback, W. (2016). Winter management. Bee Culture, October, 2016.
Jones, J.C., and Oldroyd, B.P. (2006). Nest thermoregulation in social insects. Advances in Insect Physiology, Elsevier, Volume 33:153-191.
Meikle, W., and Holst, N. (2015). Application of continuous monitoring of honeybee colonies. Apidologie, Springer Verlag, 46 (1):10-22.
Morgan, G. A., Leech, N. L., Gloeckner, G. W., and Barrett, K. C. (2011). IBM SPSS for Introductory Statistics: Use and Interpretation, 4th Edition. New York: Routledge Taylor and Francis Group.
Shaw, J.A., Nugent, P.W., Johnson, J., Bromenshenk, J.J., Henderson, C.B., and Debnam, S. (2011). Long-wave infrared imaging for non-invasive beehive population assessment. Optics express 19(1):399-408.
Southwick, E.E., Heldmaier, G. (1987).Temperature control in honey bee colonies. BioScience 37(6):395-399.
Storch, H. (1985). At the Hive Entrance (F. Celis, Trans.). European Apicultural Editions, Brussels, Belgium.
